Dynamin-2 function and dysfunction along the secretory pathway
- PMID: 24065954
- PMCID: PMC3776141
- DOI: 10.3389/fendo.2013.00126
Dynamin-2 function and dysfunction along the secretory pathway
Abstract
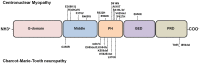
Dynamin-2 is a ubiquitously expressed mechano-GTPase involved in different stages of the secretory pathway. Its most well-known function relates to the scission of nascent vesicles from the plasma membrane during endocytosis; however, it also participates in the formation of new vesicles from the Golgi network, vesicle trafficking, fusion processes and in the regulation of microtubule, and actin cytoskeleton dynamics. Over the last 8 years, more than 20 mutations in the dynamin-2 gene have been associated to two hereditary neuromuscular disorders: Charcot-Marie-Tooth neuropathy and centronuclear myopathy. Most of these mutations are grouped in the pleckstrin homology domain; however, there are no common mutations associated with both disorders, suggesting that they differently impact on dynamin-2 function in diverse tissues. In this review, we discuss the impact of these disease-related mutations on dynamin-2 function during vesicle trafficking and endocytotic processes.
Keywords: Charcot–Marie–Tooth neuropathy; actin; centronuclear myopathy; dynamin-2; endocytosis; exocytosis; microtubules; mutations.
Figures
Similar articles
-
Dynamin 2 and human diseases.J Mol Med (Berl). 2010 Apr;88(4):339-50. doi: 10.1007/s00109-009-0587-4. Epub 2010 Feb 3. J Mol Med (Berl). 2010. PMID: 20127478 Review.
-
Dynamin-2 in nervous system disorders.J Neurochem. 2014 Jan;128(2):210-23. doi: 10.1111/jnc.12455. Epub 2013 Oct 23. J Neurochem. 2014. PMID: 24102355 Review.
-
Mild functional differences of dynamin 2 mutations associated to centronuclear myopathy and Charcot-Marie Tooth peripheral neuropathy.PLoS One. 2011;6(11):e27498. doi: 10.1371/journal.pone.0027498. Epub 2011 Nov 11. PLoS One. 2011. PMID: 22096584 Free PMC article.
-
Dynamin-2 mutations linked to neonatal-onset centronuclear myopathy impair exocytosis and endocytosis in adrenal chromaffin cells.J Neurochem. 2024 Sep;168(9):3268-3283. doi: 10.1111/jnc.16194. Epub 2024 Aug 10. J Neurochem. 2024. PMID: 39126680
-
Role of dynamin 2 in the disassembly of focal adhesions.J Mol Med (Berl). 2013 Jul;91(7):803-9. doi: 10.1007/s00109-013-1040-2. Epub 2013 Apr 23. J Mol Med (Berl). 2013. PMID: 23609221 Review.
Cited by
-
A defined clathrin-mediated trafficking pathway regulates sFLT1/VEGFR1 secretion from endothelial cells.Angiogenesis. 2024 Feb;27(1):67-89. doi: 10.1007/s10456-023-09893-6. Epub 2023 Sep 11. Angiogenesis. 2024. PMID: 37695358 Free PMC article.
-
A Charge Swap mutation E461K in the yeast dynamin Vps1 reduces endocytic invagination.Commun Integr Biol. 2015 Aug 31;8(4):e1051274. doi: 10.1080/19420889.2015.1051274. eCollection 2015 Jul-Aug. Commun Integr Biol. 2015. PMID: 26478779 Free PMC article.
-
The host GTPase Dynamin 2 modulates apical junction structure to control cell-to-cell spread of Listeria monocytogenes.Infect Immun. 2024 Oct 15;92(10):e0013624. doi: 10.1128/iai.00136-24. Epub 2024 Aug 12. Infect Immun. 2024. PMID: 39133017
-
Antiviral Mx proteins have an ancient origin and widespread distribution among eukaryotes.bioRxiv [Preprint]. 2024 Aug 17:2024.08.06.606855. doi: 10.1101/2024.08.06.606855. bioRxiv. 2024. PMID: 39149278 Free PMC article. Preprint.
-
Organization of a cytoskeletal superstructure in the apical domain of intestinal tuft cells.J Cell Biol. 2024 Dec 2;223(12):e202404070. doi: 10.1083/jcb.202404070. Epub 2024 Oct 1. J Cell Biol. 2024. PMID: 39352498 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

