Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology
- PMID: 24064666
- PMCID: PMC3965659
- DOI: 10.1038/mi.2013.71
Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology
Abstract
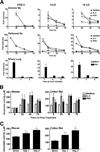
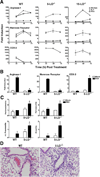
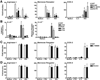
Resolution of severe Respiratory Syncytial Virus (RSV)-induced bronchiolitis is mediated by alternatively activated macrophages (AA-Mφ) that counteract cyclooxygenase (COX)-2-induced lung pathology. Herein, we report that RSV infection of 5-lipoxygenase (LO)(-/-) and 15-LO(-/-) macrophages or mice failed to elicit AA-Mφ differentiation and concomitantly exhibited increased COX-2 expression. Further, RSV infection of 5-LO(-/-) mice resulted in enhanced lung pathology. Pharmacologic inhibition of 5-LO or 15-LO also blocked differentiation of RSV-induced AA-Mφ in vitro and, conversely, treatment of 5-LO(-/-) macrophages with downstream products, lipoxin A4 and resolvin E1, but not leukotriene B4 or leukotriene D4, partially restored expression of AA-Mφ markers. Indomethacin blockade of COX activity in RSV-infected macrophages increased 5-LO and 15-LO, as well as arginase-1 mRNA expression. Treatment of RSV-infected mice with indomethacin also resulted not only in enhanced lung arginase-1 mRNA expression and decreased COX-2, but also decreased lung pathology in RSV-infected 5-LO(-/-) mice. Treatment of RSV-infected cotton rats with a COX-2-specific inhibitor resulted in enhanced lung 5-LO mRNA and AA-Mφ marker expression. Together, these data suggest a novel therapeutic approach for RSV that promotes AA-Mφ differentiation by activating the 5-LO pathway.
Conflict of interest statement
Disclosure: The authors have no conflict of interest to declare.
Figures

Similar articles
-
Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent.Mucosal Immunol. 2010 May;3(3):291-300. doi: 10.1038/mi.2010.6. Epub 2010 Mar 10. Mucosal Immunol. 2010. PMID: 20404812 Free PMC article.
-
Arachidonic acid metabolites follow the preferential course of cyclooxygenase pathway for the basal tone in the internal anal sphincter.Am J Physiol Gastrointest Liver Physiol. 2009 Apr;296(4):G727-34. doi: 10.1152/ajpgi.90707.2008. Epub 2009 Feb 12. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19221012 Free PMC article.
-
Dual 12/15- and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in ApoE knock-out mice.J Biol Chem. 2009 Jul 31;284(31):21077-89. doi: 10.1074/jbc.M109.000901. Epub 2009 Jun 9. J Biol Chem. 2009. PMID: 19509298 Free PMC article.
-
Inhibition of 5-lipoxygenase pathway attenuates acute liver failure by inhibiting macrophage activation.J Immunol Res. 2014;2014:697560. doi: 10.1155/2014/697560. Epub 2014 Jun 1. J Immunol Res. 2014. PMID: 24987711 Free PMC article.
-
Engystol reduces onset of experimental respiratory syncytial virus-induced respiratory inflammation in mice by modulating macrophage phagocytic capacity.PLoS One. 2018 Apr 19;13(4):e0195822. doi: 10.1371/journal.pone.0195822. eCollection 2018. PLoS One. 2018. PMID: 29672626 Free PMC article.
Cited by
-
The Prostaglandin E2-EP3 Receptor Axis Regulates Anaplasma phagocytophilum-Mediated NLRC4 Inflammasome Activation.PLoS Pathog. 2016 Aug 2;12(8):e1005803. doi: 10.1371/journal.ppat.1005803. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27482714 Free PMC article.
-
Respiratory viral infection and resolution of inflammation: Roles for specialized pro-resolving mediators.Exp Biol Med (Maywood). 2023 Oct;248(19):1635-1644. doi: 10.1177/15353702231199082. Epub 2023 Oct 14. Exp Biol Med (Maywood). 2023. PMID: 37837390 Free PMC article. Review.
-
Enhanced allergic responsiveness after early childhood infection with respiratory viruses: Are long-lived alternatively activated macrophages the missing link?Pathog Dis. 2016 Jul;74(5):ftw047. doi: 10.1093/femspd/ftw047. Epub 2016 May 12. Pathog Dis. 2016. PMID: 27178560 Free PMC article.
-
A metabolic associated fatty liver disease risk variant in MBOAT7 regulates toll like receptor induced outcomes.Nat Commun. 2022 Dec 6;13(1):7430. doi: 10.1038/s41467-022-35158-9. Nat Commun. 2022. PMID: 36473860 Free PMC article.
-
Specialized pro-resolving lipid mediators regulate inflammatory macrophages: A paradigm shift from antibiotics to immunotherapy for mitigating COVID-19 pandemic.Front Mol Biosci. 2023 Feb 3;10:1104577. doi: 10.3389/fmolb.2023.1104577. eCollection 2023. Front Mol Biosci. 2023. PMID: 36825200 Free PMC article. Review.
References
-
- Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–S117. - PubMed
-
- IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. - PubMed
-
- Richardson JY, et al. Respiratory syncytial virus (RSV) infection induces cyclooxygenase 2: a potential target for RSV therapy. J. Immunol. 2005;174:4356–4364. - PubMed
-
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

