The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging
- PMID: 24062640
- PMCID: PMC3775451
- DOI: 10.3389/fncel.2013.00147
The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging
Abstract
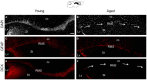
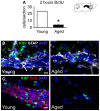
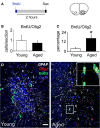
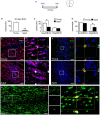
The subventricular zone (SVZ) is the largest source of newly generated cells in the adult mammalian brain. SVZ-derived neuroblasts migrate via the rostral migratory stream (RMS) to the olfactory bulb (OB), where they differentiate into mature neurons. Additionally, a small proportion of SVZ-derived cells contribute to the generation of myelinating oligodendrocytes. The production of new cells in the SVZ decreases during aging, affecting the incorporation of new neurons into the OB. However, the age-related changes that occur across the RMS are not fully understood. In this study we evaluate how aging affects the cellular organization of migrating neuroblast chains, the proliferation, and the fate of the newly generated cells in the SVZ-OB system. By using electron microscopy and immunostaining, we found that the RMS path becomes discontinuous and its cytoarchitecture is disorganized in aged mice (24-month-old mice). Subsequently, OB neurogenesis was impaired in the aged brain while the production of oligodendrocytes was not compromised. These findings provide new insight into oligodendrocyte preservation throughout life. Further exploration of this matter could help the development of new strategies to prevent neurological disorders associated with senescence.
Keywords: aging; neuroblast migration; neurogenesis; olfactory bulb; oligodendrogenesis; rostral migratory stream; subventricular zone.
Figures


Similar articles
-
Age-dependent regional changes in the rostral migratory stream.Neurobiol Aging. 2013 Jul;34(7):1873-81. doi: 10.1016/j.neurobiolaging.2013.01.015. Epub 2013 Feb 15. Neurobiol Aging. 2013. PMID: 23419702 Free PMC article.
-
EphA4 Regulates Neuroblast and Astrocyte Organization in a Neurogenic Niche.J Neurosci. 2017 Mar 22;37(12):3331-3341. doi: 10.1523/JNEUROSCI.3738-16.2017. Epub 2017 Mar 3. J Neurosci. 2017. PMID: 28258169 Free PMC article.
-
Age-related impairment of olfactory bulb neurogenesis in the Ts65Dn mouse model of Down syndrome.Exp Neurol. 2014 Jan;251:1-11. doi: 10.1016/j.expneurol.2013.10.018. Epub 2013 Nov 2. Exp Neurol. 2014. PMID: 24192151
-
The aged brain: genesis and fate of residual progenitor cells in the subventricular zone.Front Cell Neurosci. 2015 Sep 24;9:365. doi: 10.3389/fncel.2015.00365. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26441536 Free PMC article. Review.
-
Regulation of subventricular zone-derived cells migration in the adult brain.Adv Exp Med Biol. 2015;853:1-21. doi: 10.1007/978-3-319-16537-0_1. Adv Exp Med Biol. 2015. PMID: 25895704 Review.
Cited by
-
Niche derived oligodendrocyte progenitors: a source of rejuvenation or complementation for local oligodendrogenesis?Front Cell Neurosci. 2013 Oct 22;7:188. doi: 10.3389/fncel.2013.00188. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24155691 Free PMC article. No abstract available.
-
Promoting Myelin Repair through In Vivo Neuroblast Reprogramming.Stem Cell Reports. 2018 May 8;10(5):1492-1504. doi: 10.1016/j.stemcr.2018.02.015. Epub 2018 Mar 29. Stem Cell Reports. 2018. PMID: 29606615 Free PMC article.
-
Thyroid Hormone and Neural Stem Cells: Repair Potential Following Brain and Spinal Cord Injury.Front Neurosci. 2020 Aug 26;14:875. doi: 10.3389/fnins.2020.00875. eCollection 2020. Front Neurosci. 2020. PMID: 32982671 Free PMC article. Review.
-
Aging in the olfactory system.Trends Neurosci. 2014 Feb;37(2):77-84. doi: 10.1016/j.tins.2013.11.004. Epub 2013 Dec 19. Trends Neurosci. 2014. PMID: 24361044 Free PMC article. Review.
-
Isolation of neural stem and oligodendrocyte progenitor cells from the brain of live rats.Stem Cell Reports. 2021 Oct 12;16(10):2534-2547. doi: 10.1016/j.stemcr.2021.08.015. Epub 2021 Sep 23. Stem Cell Reports. 2021. PMID: 34560001 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

