Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections
- PMID: 24061146
- PMCID: PMC4049945
- DOI: 10.4161/gmic.26419
Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections
Abstract
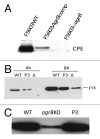
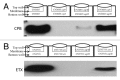
Clostridium perfringens causes enteritis and enterotoxemia in humans and livestock due to prolific toxin production. In broth culture, C. perfringens uses the Agr-like quorum sensing (QS) system to regulate production of toxins important for enteritis/enterotoxemia, including beta toxin (CPB), enterotoxin, and epsilon toxin (ETX). The VirS/VirR two-component regulatory system (TCRS) also controls CPB production in broth cultures. Both the Agr-like QS and VirS/VirR systems are important when C. perfringens senses enterocyte-like Caco-2 cells and responds by upregulating CPB production; however, only the Agr-like QS system is needed for host cell-induced ETX production. These in vitro observations have pathophysiologic relevance since both the VirS/VirR and Agr-like QS signaling systems are required for C. perfringens strain CN3685 to produce CPB in vivo and to cause enteritis or enterotoxemia. Thus, apparently upon sensing its presence in the intestines, C. perfringens utilizes QS and TCRS signaling to produce toxins necessary for intestinal virulence.
Keywords: Agr; Clostridium perfringens; VirS/VirR; beta toxin; enterotoxin; epsilon toxin; intestinal infection; quorum sensing; two component regulatory system.
Figures


Similar articles
-
Epsilon-toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two-component regulatory system.mBio. 2011 Dec 13;2(6):e00275-11. doi: 10.1128/mBio.00275-11. Print 2011. mBio. 2011. Retraction in: mBio. 2022 Apr 26;13(2):e0049522. doi: 10.1128/mbio.00495-22. PMID: 22167225 Free PMC article. Retracted.
-
Identifying the Basis for VirS/VirR Two-Component Regulatory System Control of Clostridium perfringens Beta-Toxin Production.J Bacteriol. 2021 Aug 20;203(18):e0027921. doi: 10.1128/JB.00279-21. Epub 2021 Aug 20. J Bacteriol. 2021. PMID: 34228498 Free PMC article.
-
Evidence That VirS Is a Receptor for the Signaling Peptide of the Clostridium perfringens Agr-like Quorum Sensing System.mBio. 2020 Sep 15;11(5):e02219-20. doi: 10.1128/mBio.02219-20. mBio. 2020. PMID: 32934089 Free PMC article.
-
Gene regulation by the VirS/VirR system in Clostridium perfringens.Anaerobe. 2016 Oct;41:5-9. doi: 10.1016/j.anaerobe.2016.06.003. Epub 2016 Jun 11. Anaerobe. 2016. PMID: 27296833 Review.
-
Regulation of toxin gene expression in Clostridium perfringens.Res Microbiol. 2015 May;166(4):280-9. doi: 10.1016/j.resmic.2014.09.010. Epub 2014 Oct 7. Res Microbiol. 2015. PMID: 25303832 Review.
Cited by
-
The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of Clostridium perfringens ε-Toxin.PLoS Pathog. 2015 May 20;11(5):e1004896. doi: 10.1371/journal.ppat.1004896. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25993478 Free PMC article.
-
Effect of Clostridium perfringens β-Toxin on Platelets.Toxins (Basel). 2017 Oct 24;9(10):336. doi: 10.3390/toxins9100336. Toxins (Basel). 2017. PMID: 29064418 Free PMC article.
-
Regulation of Toxin Production in Clostridium perfringens.Toxins (Basel). 2016 Jul 5;8(7):207. doi: 10.3390/toxins8070207. Toxins (Basel). 2016. PMID: 27399773 Free PMC article. Review.
-
Structure-function analysis of peptide signaling in the Clostridium perfringens Agr-like quorum sensing system.J Bacteriol. 2015 May;197(10):1807-18. doi: 10.1128/JB.02614-14. Epub 2015 Mar 16. J Bacteriol. 2015. PMID: 25777675 Free PMC article.
-
In this issue of Gut Microbes.Gut Microbes. 2014 Jan-Feb;5(1):83-5. doi: 10.4161/gmic.28007. Epub 2014 Jan 27. Gut Microbes. 2014. PMID: 24468723 Free PMC article. No abstract available.
References
-
- McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. (2006) The Enterotoxic Clostridia. In: Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E, editors. The Prokaryotes. 3rd ed. New York: Springer NY press. pp. 688-752.
-
- McClane BA, Robertson SL, Li J. (2013) Clostridium perfringens. In: Doyle MP, Buchanan RL, editors. Food Microbiology: Fundamentals and Frontiers. 4th ed. Washington D.C.: ASM press. pp. 465-489.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
