Reduction of synaptojanin 1 accelerates Aβ clearance and attenuates cognitive deterioration in an Alzheimer mouse model
- PMID: 24052255
- PMCID: PMC3814799
- DOI: 10.1074/jbc.M113.504365
Reduction of synaptojanin 1 accelerates Aβ clearance and attenuates cognitive deterioration in an Alzheimer mouse model
Abstract
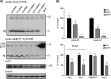
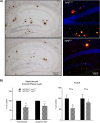
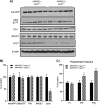
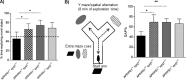
Recent studies link synaptojanin 1 (synj1), the main phosphoinositol (4,5)-biphosphate phosphatase (PI(4,5)P2-degrading enzyme) in the brain and synapses, to Alzheimer disease. Here we report a novel mechanism by which synj1 reversely regulates cellular clearance of amyloid-β (Aβ). Genetic down-regulation of synj1 reduces both extracellular and intracellular Aβ levels in N2a cells stably expressing the Swedish mutant of amyloid precursor protein (APP). Moreover, synj1 haploinsufficiency in an Alzheimer disease transgenic mouse model expressing the Swedish mutant APP and the presenilin-1 mutant ΔE9 reduces amyloid plaque load, as well as Aβ40 and Aβ42 levels in hippocampus of 9-month-old animals. Reduced expression of synj1 attenuates cognitive deficits in these transgenic mice. However, reduction of synj1 does not affect levels of full-length APP and the C-terminal fragment, suggesting that Aβ generation by β- and γ-secretase cleavage is not affected. Instead, synj1 knockdown increases Aβ uptake and cellular degradation through accelerated delivery to lysosomes. These effects are partially dependent upon elevated PI(4,5)P2 with synj1 down-regulation. In summary, our data suggest a novel mechanism by which reduction of a PI(4,5)P2-degrading enzyme, synj1, improves amyloid-induced neuropathology and behavior deficits through accelerating cellular Aβ clearance.
Keywords: Alzheimer Disease; Amyloid; Clearance; Endosomal/Lysosomal Degradation; Intracellular Trafficking; PIP2; Protein Degradation; Synaptojanin 1; Transgenic Mice.
Figures


Similar articles
-
Reduction of synaptojanin 1 ameliorates synaptic and behavioral impairments in a mouse model of Alzheimer's disease.J Neurosci. 2012 Oct 31;32(44):15271-6. doi: 10.1523/JNEUROSCI.2034-12.2012. J Neurosci. 2012. PMID: 23115165 Free PMC article.
-
Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism.Nat Neurosci. 2008 May;11(5):547-54. doi: 10.1038/nn.2100. Epub 2008 Apr 6. Nat Neurosci. 2008. PMID: 18391946 Free PMC article.
-
Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Aβ Generation and Amyloid Plaque Pathogenesis.J Neurosci. 2015 Sep 2;35(35):12137-51. doi: 10.1523/JNEUROSCI.0705-15.2015. J Neurosci. 2015. PMID: 26338325 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Amyloid beta-protein toxicity and the pathogenesis of Alzheimer disease.J Biol Chem. 2009 Feb 20;284(8):4755-9. doi: 10.1074/jbc.R800018200. Epub 2008 Oct 28. J Biol Chem. 2009. PMID: 18957434 Free PMC article. Review. No abstract available.
Cited by
-
Phosphoinositides: Roles in the Development of Microglial-Mediated Neuroinflammation and Neurodegeneration.Front Cell Neurosci. 2021 Mar 26;15:652593. doi: 10.3389/fncel.2021.652593. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33841102 Free PMC article. Review.
-
MicroRNA-195 rescues ApoE4-induced cognitive deficits and lysosomal defects in Alzheimer's disease pathogenesis.Mol Psychiatry. 2021 Sep;26(9):4687-4701. doi: 10.1038/s41380-020-0824-3. Epub 2020 Jul 6. Mol Psychiatry. 2021. PMID: 32632205 Free PMC article.
-
Mechanistic Analysis of Age-Related Clinical Manifestations in Down Syndrome.Front Aging Neurosci. 2021 Jul 1;13:700280. doi: 10.3389/fnagi.2021.700280. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34276349 Free PMC article.
-
Synaptojanin 1 mutation in Parkinson's disease brings further insight into the neuropathological mechanisms.Biomed Res Int. 2014;2014:289728. doi: 10.1155/2014/289728. Epub 2014 Sep 16. Biomed Res Int. 2014. PMID: 25302295 Free PMC article. Review.
-
Endo-lysosomal pathway and ubiquitin-proteasome system dysfunction in Alzheimer's disease pathogenesis.Neurosci Lett. 2019 Jun 11;703:68-78. doi: 10.1016/j.neulet.2019.03.016. Epub 2019 Mar 16. Neurosci Lett. 2019. PMID: 30890471 Free PMC article. Review.
References
-
- Cremona O., Di Paolo G., Wenk M. R., Lüthi A., Kim W. T., Takei K., Daniell L., Nemoto Y., Shears S. B., Flavell R. A., McCormick D. A., De Camilli P. (1999) Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179–188 - PubMed
-
- Voronov S. V., Frere S. G., Giovedi S., Pollina E. A., Borel C., Zhang H., Schmidt C., Akeson E. C., Wenk M. R., Cimasoni L., Arancio O., Davisson M. T., Antonarakis S. E., Gardiner K., De Camilli P., Di Paolo G. (2008) Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome. Proc. Natl. Acad. Sci. U.S.A. 105, 9415–9420 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

