The interplay between cell signalling and mechanics in developmental processes
- PMID: 24045690
- PMCID: PMC4056017
- DOI: 10.1038/nrg3513
The interplay between cell signalling and mechanics in developmental processes
Abstract
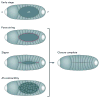
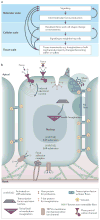
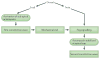
Force production and the propagation of stress and strain within embryos and organisms are crucial physical processes that direct morphogenesis. In addition, there is mounting evidence that biomechanical cues created by these processes guide cell behaviours and cell fates. In this Review we discuss key roles for biomechanics during development to directly shape tissues, to provide positional information for cell fate decisions and to enable robust programmes of development. Several recently identified molecular mechanisms suggest how cells and tissues might coordinate their responses to biomechanical cues. Finally, we outline long-term challenges in integrating biomechanics with genetic analysis of developing embryos.
Figures
Similar articles
-
Embryo mechanics: balancing force production with elastic resistance during morphogenesis.Curr Top Dev Biol. 2011;95:215-41. doi: 10.1016/B978-0-12-385065-2.00007-4. Curr Top Dev Biol. 2011. PMID: 21501753 Review.
-
Mechanical control of tissue and organ development.Development. 2010 May;137(9):1407-20. doi: 10.1242/dev.024166. Development. 2010. PMID: 20388652 Free PMC article. Review.
-
[The irruption of mechanics in the chemistry of life].Med Sci (Paris). 2018 Nov;34(11):963-971. doi: 10.1051/medsci/2018241. Epub 2018 Dec 10. Med Sci (Paris). 2018. PMID: 30526840 Review. French.
-
Interplay between mechanics and signalling in regulating cell fate.Nat Rev Mol Cell Biol. 2022 Jul;23(7):465-480. doi: 10.1038/s41580-022-00472-z. Epub 2022 Apr 1. Nat Rev Mol Cell Biol. 2022. PMID: 35365816 Review.
-
Mechanical design in embryos: mechanical signalling, robustness and developmental defects.Philos Trans R Soc Lond B Biol Sci. 2017 May 19;372(1720):20150516. doi: 10.1098/rstb.2015.0516. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28348252 Free PMC article. Review.
Cited by
-
Hydraulic fracture during epithelial stretching.Nat Mater. 2015 Mar;14(3):343-51. doi: 10.1038/nmat4206. Epub 2015 Feb 9. Nat Mater. 2015. PMID: 25664452 Free PMC article.
-
Brillouin microscopy.Nat Rev Methods Primers. 2024;4:8. doi: 10.1038/s43586-023-00286-z. Epub 2024 Feb 1. Nat Rev Methods Primers. 2024. PMID: 39391288 Free PMC article.
-
The Cell as Matter: Connecting Molecular Biology to Cellular Functions.Matter. 2021 Jun 2;4(6):1863-1891. doi: 10.1016/j.matt.2021.03.013. Matter. 2021. PMID: 35495565 Free PMC article.
-
Viscoelastic biomarker for differentiation of benign and malignant breast lesion in ultra- low frequency range.Sci Rep. 2019 Apr 5;9(1):5737. doi: 10.1038/s41598-019-41885-9. Sci Rep. 2019. PMID: 30952880 Free PMC article.
-
Socket Array Irregularities and Wing Membrane Distortions at the Eyespot Foci of Butterfly Wings Suggest Mechanical Signals for Color Pattern Determination.Insects. 2024 Jul 16;15(7):535. doi: 10.3390/insects15070535. Insects. 2024. PMID: 39057268 Free PMC article.
References
-
- His W. On the principles of animal morphology. Proceedings of Royal Society of Edinburgh. 1888;15:287–298.
-
- Rhumbler L. Zur mechanik des gastrulations vorganges insbesondere der invagination. Archiv Fur Entwicklungs mechanic. 1902;14:401–476.
-
- Morgan TH. Experimental Embryology. Columbia University Press; 1927.
-
- Lewis WH. Mechanics of Invagination. Anatomical Record. 1947;97:139–56. - PubMed
-
- Howard J. Mechanics of Motor Proteins and the Cytoskeleton. Sinauer Associates; Sunderland, MA: 2001. p. 367.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

