Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling
- PMID: 24041503
- PMCID: PMC3944379
- DOI: 10.1016/j.biopsych.2013.07.033
Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling
Abstract
Background: Refinement of mature cognitive functions, such as working memory and decision making, typically takes place during adolescence. The acquisition of these functions is linked to the protracted development of the prefrontal cortex (PFC) and dopamine facilitation of glutamatergic transmission. However, the mechanisms that support these changes during adolescence remain elusive.
Methods: Electrophysiological recordings (in vitro and in vivo) combined with pharmacologic manipulations were employed to determine how N-methyl-D-aspartate transmission in the medial PFC changes during the adolescent transition to adulthood. The relative contribution of GluN2B transmission and its modulation by postsynaptic protein kinase A and D1 receptor signaling were determined in two distinct age groups of rats: postnatal day (P)25 to P40 and P50 to P80.
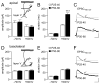
Results: We found that only N-methyl-D-aspartate receptor transmission onto the apical dendrite of layer V pyramidal neurons undergoes late adolescent remodeling due to a functional emergence of GluN2B function after P40. Both protein kinase A and dopamine D1 receptor signaling are required for the functional expression of GluN2B transmission and to sustain PFC plasticity in response to ventral hippocampal, but not basolateral amygdala, inputs.
Conclusions: Thus, the late adolescent acquisition of GluN2B function provides a mechanism for dopamine D1-mediated regulation of PFC responses in an input-specific manner.
Keywords: Adolescence; NMDA; amygdala; dopamine; hippocampus; signaling.
Copyright © 2014 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
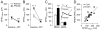
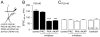
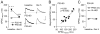
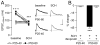
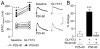


Similar articles
-
Concurrent upregulation of postsynaptic L-type Ca(2+) channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca(2+) plateau potentials and dopamine D1 receptor modulation in the prefrontal cortex.Neuropharmacology. 2011 May;60(6):953-62. doi: 10.1016/j.neuropharm.2011.01.041. Epub 2011 Feb 1. Neuropharmacology. 2011. PMID: 21288471 Free PMC article.
-
Dopaminergic enhancement of excitatory synaptic transmission in layer II entorhinal neurons is dependent on D₁-like receptor-mediated signaling.Neuroscience. 2014 Jan 31;258:74-83. doi: 10.1016/j.neuroscience.2013.10.076. Epub 2013 Nov 9. Neuroscience. 2014. PMID: 24220689
-
Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence.Psychopharmacology (Berl). 2014 Apr;231(8):1789-96. doi: 10.1007/s00213-013-3216-4. Epub 2013 Aug 2. Psychopharmacology (Berl). 2014. PMID: 23907651 Free PMC article.
-
PET neuroimaging of extrastriatal dopamine receptors and prefrontal cortex functions.J Physiol Paris. 2013 Dec;107(6):503-9. doi: 10.1016/j.jphysparis.2013.07.001. Epub 2013 Jul 12. J Physiol Paris. 2013. PMID: 23851135 Review.
-
Under the curve: critical issues for elucidating D1 receptor function in working memory.Neuroscience. 2006 Apr 28;139(1):263-76. doi: 10.1016/j.neuroscience.2005.09.028. Epub 2005 Nov 28. Neuroscience. 2006. PMID: 16310964 Review.
Cited by
-
Mechanisms contributing to prefrontal cortex maturation during adolescence.Neurosci Biobehav Rev. 2016 Nov;70:4-12. doi: 10.1016/j.neubiorev.2016.05.013. Epub 2016 May 24. Neurosci Biobehav Rev. 2016. PMID: 27235076 Free PMC article. Review.
-
Adolescent thalamic inhibition leads to long-lasting impairments in prefrontal cortex function.Nat Neurosci. 2022 Jun;25(6):714-725. doi: 10.1038/s41593-022-01072-y. Epub 2022 May 19. Nat Neurosci. 2022. PMID: 35590075 Free PMC article.
-
Maturation of Corticolimbic Functional Connectivity During Sensitive Periods of Brain Development.Curr Top Behav Neurosci. 2022;53:37-53. doi: 10.1007/7854_2021_239. Curr Top Behav Neurosci. 2022. PMID: 34386969
-
Late-adolescent onset of prefrontal endocannabinoid control of hippocampal and amygdalar inputs and its impact on trace-fear conditioning behavior.Neuropsychopharmacology. 2024 Aug;49(9):1417-1424. doi: 10.1038/s41386-024-01844-z. Epub 2024 Mar 11. Neuropsychopharmacology. 2024. PMID: 38467844
-
The role of dopamine and endocannabinoid systems in prefrontal cortex development: Adolescence as a critical period.Front Neural Circuits. 2022 Nov 1;16:939235. doi: 10.3389/fncir.2022.939235. eCollection 2022. Front Neural Circuits. 2022. PMID: 36389180 Free PMC article. Review.
References
-
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. - PubMed
-
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. - PubMed
-
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

