Fibrillin-1 directly regulates osteoclast formation and function by a dual mechanism
- PMID: 24039232
- PMCID: PMC4961468
- DOI: 10.1242/jcs.127571
Fibrillin-1 directly regulates osteoclast formation and function by a dual mechanism
Abstract
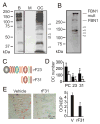
Mutations in the fibrillin-1 gene give rise to a number of heritable disorders, which are all characterized by various malformations of bone as well as manifestations in other tissues. However, the role of fibrillin-1 in the development and homeostasis of bone is not well understood. Here, we examined the role of fibrillin-1 in regulating osteoclast differentiation from primary bone-marrow-derived precursors and monocytic RAW 264.7 cells. The soluble N-terminal half of fibrillin-1 (rFBN1-N) strongly inhibited osteoclastogenesis, whereas the C-terminal half (rFBN1-C) did not. By contrast, when rFBN1-N was immobilized on calcium phosphate, it did not affect osteoclastogenesis but modulated osteoclast resorptive activity, which was evident by a larger number of smaller resorption pits. Using a panel of recombinant sub-fragments spanning rFBN1-N, we localized an osteoclast inhibitory activity to the 63 kDa subfragment rF23 comprising the N-terminal region of fibrillin-1. Osteoclastic resorption led to the generation of small fibrillin-1 fragments that were similar to those identified in human vertebral bone extracts. rF23, but not rFBN1-N, was found to inhibit the expression of cathepsin K, matrix metalloproteinase 9 and Dcstamp in differentiating osteoclasts. rFBN1-N, but not rF23, exhibited interaction with RANKL. Excess RANKL rescued the inhibition of osteoclastogenesis by rFBN1-N. By contrast, rF23 disrupted RANKL-induced Ca(2+) signaling and activation of transcription factor NFATc1. These studies highlight a direct dual inhibitory role of N-terminal fibrillin-1 fragments in osteoclastogenesis, the sequestration of RANKL and the inhibition of NFATc1 signaling, demonstrating that osteoclastic degradation of fibrillin-1 provides a potent negative feedback that limits osteoclast formation and function.
Keywords: Calcium signaling; Fibrillin; NFATc1; Osteoclastogenesis; RANKL.
Figures


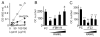

Similar articles
-
BSP and RANKL induce osteoclastogenesis and bone resorption synergistically.J Bone Miner Res. 2005 Sep;20(9):1669-79. doi: 10.1359/JBMR.050511. Epub 2005 May 16. J Bone Miner Res. 2005. PMID: 16059638
-
Microtubule actin crosslinking factor 1 (MACF1) knockdown inhibits RANKL-induced osteoclastogenesis via Akt/GSK3β/NFATc1 signalling pathway.Mol Cell Endocrinol. 2019 Aug 20;494:110494. doi: 10.1016/j.mce.2019.110494. Epub 2019 Jun 28. Mol Cell Endocrinol. 2019. PMID: 31260729
-
Inhibition of matrix metalloproteinase-9 activity by doxycycline ameliorates RANK ligand-induced osteoclast differentiation in vitro and in vivo.Exp Cell Res. 2011 Jun 10;317(10):1454-64. doi: 10.1016/j.yexcr.2011.03.014. Epub 2011 Mar 21. Exp Cell Res. 2011. PMID: 21420951 Free PMC article.
-
CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo.Bone. 2015 Oct;79:242-51. doi: 10.1016/j.bone.2015.06.011. Epub 2015 Jun 21. Bone. 2015. PMID: 26103094
-
Osteoclast differentiation by RANKL and OPG signaling pathways.J Bone Miner Metab. 2021 Jan;39(1):19-26. doi: 10.1007/s00774-020-01162-6. Epub 2020 Oct 20. J Bone Miner Metab. 2021. PMID: 33079279 Review.
Cited by
-
CD34+ cell atlas of main organs implicates its impact on fibrosis.Cell Mol Life Sci. 2022 Oct 31;79(11):576. doi: 10.1007/s00018-022-04606-6. Cell Mol Life Sci. 2022. PMID: 36315271
-
The protocol for the isolation and cryopreservation of osteoclast precursors from mouse bone marrow and spleen.Cytotechnology. 2016 Jan;68(1):105-114. doi: 10.1007/s10616-014-9759-3. Epub 2014 Sep 23. Cytotechnology. 2016. PMID: 25245056 Free PMC article.
-
Fibrillin-1 and fibrillin-1-derived asprosin in adipose tissue function and metabolic disorders.J Cell Commun Signal. 2020 Jun;14(2):159-173. doi: 10.1007/s12079-020-00566-3. Epub 2020 Apr 12. J Cell Commun Signal. 2020. PMID: 32279186 Free PMC article. Review.
-
Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFβ signaling and cell stemness.Matrix Biol. 2018 Oct;71-72:82-89. doi: 10.1016/j.matbio.2017.07.004. Epub 2017 Aug 4. Matrix Biol. 2018. PMID: 28782645 Free PMC article. Review.
-
Human perivascular stem cells prevent bone graft resorption in osteoporotic contexts by inhibiting osteoclast formation.Stem Cells Transl Med. 2020 Dec;9(12):1617-1630. doi: 10.1002/sctm.20-0152. Epub 2020 Jul 22. Stem Cells Transl Med. 2020. PMID: 32697440 Free PMC article.
References
-
- Ariyoshi W, Takahashi T, Kanno T, Ichimiya H, Shinmyouzu K, Takano H, Koseki T, Nishihara T. Heparin inhibits osteoclastic differentiation and function. J Cell Biochem. 2008;103:1707–1717. - PubMed
-
- Balkan W, Martinez AF, Fernandez I, Rodriguez MA, Pang M, Troen BR. Identification of NFAT binding sites that mediate stimulation of cathepsin K promoter activity by RANK ligand. Gene. 2009;446:90–98. - PubMed
-
- Carter N, Duncan E, Wordsworth P. Bone mineral density in adults with Marfan syndrome. Rheumatology (Oxford) 2000;39:307–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

