The REIL1 and REIL2 proteins of Arabidopsis thaliana are required for leaf growth in the cold
- PMID: 24038679
- PMCID: PMC3850186
- DOI: 10.1104/pp.113.223925
The REIL1 and REIL2 proteins of Arabidopsis thaliana are required for leaf growth in the cold
Abstract
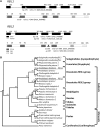
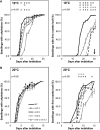
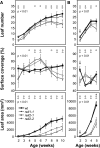
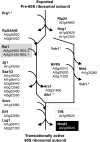
The evolutionarily conserved proteins REI1-LIKE (REIL1) and REIL2 have four conserved zinc finger domains and are Arabidopsis thaliana homologs of the cytosolic 60S ribosomal maturation factor Rei1p (for Required for isotropic bud growth1 protein) from yeast (Saccharomyces cerevisiae) and its paralog Reh1p (for REI1 homologue1 protein). The yeast and A. thaliana paralogs result from independent gene duplications. The A. thaliana REIL paralogs are required specifically in the cold (10°C) but not for growth at optimal temperature (20°C). A reil1-1 reil2-1 double mutant is arrested at 10°C prior to the emergence of the first rosette leaf. Two allelic reil2 mutants, reil2-1 and reil2-2, form small spoon-shaped leaves at 10°C. This phenomenon reverts after emergence of the inflorescence in the cold or upon shift to 20°C. Except for a slightly delayed germination, a reil1-1 mutant shows no further growth phenotype under the currently investigated conditions. A comparative analysis demonstrates conserved coexpression of orthologous genes from yeast and A. thaliana that are coregulated with yeast rei1 or with A. thaliana REIL2, respectively. The conserved correlations point to a role of A. thaliana REIL proteins in the maturation of the eukaryotic ribosomal 60S subunit. We support this conclusion by heterologous complementation of the cold-induced growth defect of the yeast Δrei1 deletion.
Figures


Similar articles
-
Integration of relative metabolomics and transcriptomics time-course data in a metabolic model pinpoints effects of ribosome biogenesis defects on Arabidopsis thaliana metabolism.Sci Rep. 2021 Feb 26;11(1):4787. doi: 10.1038/s41598-021-84114-y. Sci Rep. 2021. PMID: 33637852 Free PMC article.
-
REIL proteins of Arabidopsis thaliana interact in yeast-2-hybrid assays with homologs of the yeast Rlp24, Rpl24A, Rlp24B, Arx1, and Jjj1 proteins.Plant Signal Behav. 2014;9(3):e28224. doi: 10.4161/psb.28224. Epub 2014 Mar 6. Plant Signal Behav. 2014. PMID: 24603461 Free PMC article.
-
Plant Temperature Acclimation and Growth Rely on Cytosolic Ribosome Biogenesis Factor Homologs.Plant Physiol. 2018 Mar;176(3):2251-2276. doi: 10.1104/pp.17.01448. Epub 2018 Jan 30. Plant Physiol. 2018. PMID: 29382692 Free PMC article.
-
Arabidopsis REI-LIKE proteins activate ribosome biogenesis during cold acclimation.Sci Rep. 2021 Jan 28;11(1):2410. doi: 10.1038/s41598-021-81610-z. Sci Rep. 2021. PMID: 33510206 Free PMC article.
-
Involvement of Arabidopsis RACK1 in protein translation and its regulation by abscisic acid.Plant Physiol. 2011 Jan;155(1):370-83. doi: 10.1104/pp.110.160663. Epub 2010 Nov 19. Plant Physiol. 2011. PMID: 21098678 Free PMC article.
Cited by
-
Integration of relative metabolomics and transcriptomics time-course data in a metabolic model pinpoints effects of ribosome biogenesis defects on Arabidopsis thaliana metabolism.Sci Rep. 2021 Feb 26;11(1):4787. doi: 10.1038/s41598-021-84114-y. Sci Rep. 2021. PMID: 33637852 Free PMC article.
-
Identification of nitric oxide (NO)-responsive genes under hypoxia in tomato (Solanum lycopersicum L.) root.Sci Rep. 2020 Oct 5;10(1):16509. doi: 10.1038/s41598-020-73613-z. Sci Rep. 2020. PMID: 33020554 Free PMC article.
-
Antarctic Moss Multiprotein Bridging Factor 1c Overexpression in Arabidopsis Resulted in Enhanced Tolerance to Salt Stress.Front Plant Sci. 2017 Jul 11;8:1206. doi: 10.3389/fpls.2017.01206. eCollection 2017. Front Plant Sci. 2017. PMID: 28744295 Free PMC article.
-
Transcriptomic analysis in tomato fruit reveals divergences in genes involved in cold stress response and fruit ripening.Front Plant Sci. 2023 Jul 28;14:1227349. doi: 10.3389/fpls.2023.1227349. eCollection 2023. Front Plant Sci. 2023. PMID: 37575935 Free PMC article.
-
REIL proteins of Arabidopsis thaliana interact in yeast-2-hybrid assays with homologs of the yeast Rlp24, Rpl24A, Rlp24B, Arx1, and Jjj1 proteins.Plant Signal Behav. 2014;9(3):e28224. doi: 10.4161/psb.28224. Epub 2014 Mar 6. Plant Signal Behav. 2014. PMID: 24603461 Free PMC article.
References
-
- Agarwal P, Arora R, Ray S, Singh AK, Singh VP, Takatsuji H, Kapoor S, Tyagi AK. (2007) Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol Biol 65: 467–485 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

