Antibodies in HIV-1 vaccine development and therapy
- PMID: 24031012
- PMCID: PMC3970325
- DOI: 10.1126/science.1241144
Antibodies in HIV-1 vaccine development and therapy
Abstract
Despite 30 years of study, there is no HIV-1 vaccine and, until recently, there was little hope for a protective immunization. Renewed optimism in this area of research comes in part from the results of a recent vaccine trial and the use of single-cell antibody-cloning techniques that uncovered naturally arising, broad and potent HIV-1-neutralizing antibodies (bNAbs). These antibodies can protect against infection and suppress established HIV-1 infection in animal models. The finding that these antibodies develop in a fraction of infected individuals supports the idea that new approaches to vaccination might be developed by adapting the natural immune strategies or by structure-based immunogen design. Moreover, the success of passive immunotherapy in small-animal models suggests that bNAbs may become a valuable addition to the armamentarium of drugs that work against HIV-1.
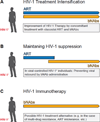
Figures



Similar articles
-
Engineered Expression of Broadly Neutralizing Antibodies Against Human Immunodeficiency Virus.Annu Rev Virol. 2017 Sep 29;4(1):491-510. doi: 10.1146/annurev-virology-101416-041929. Epub 2017 Jun 23. Annu Rev Virol. 2017. PMID: 28645240 Review.
-
AIDS/HIV. Host controls of HIV neutralizing antibodies.Science. 2014 May 9;344(6184):588-9. doi: 10.1126/science.1254990. Science. 2014. PMID: 24812389 Free PMC article.
-
Targeting B-cell germlines and focusing affinity maturation: the next hurdles in HIV-1-vaccine development?Expert Rev Vaccines. 2014 Apr;13(4):449-52. doi: 10.1586/14760584.2014.894469. Expert Rev Vaccines. 2014. PMID: 24606603 Free PMC article.
-
Broadly Neutralizing Antibodies Against HIV: New Insights to Inform Vaccine Design.Annu Rev Med. 2016;67:185-200. doi: 10.1146/annurev-med-091014-090749. Epub 2015 Nov 9. Annu Rev Med. 2016. PMID: 26565674 Review.
-
Minimally Mutated HIV-1 Broadly Neutralizing Antibodies to Guide Reductionist Vaccine Design.PLoS Pathog. 2016 Aug 25;12(8):e1005815. doi: 10.1371/journal.ppat.1005815. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27560183 Free PMC article.
Cited by
-
Oral delivery of anti-TNF antibody shielded by natural polyphenol-mediated supramolecular assembly for inflammatory bowel disease therapy.Theranostics. 2020 Aug 29;10(23):10808-10822. doi: 10.7150/thno.47601. eCollection 2020. Theranostics. 2020. PMID: 32929381 Free PMC article.
-
Can immunological principles and cross-disciplinary science illuminate the path to vaccines for HIV and other global health challenges?Philos Trans R Soc Lond B Biol Sci. 2015 Jun 19;370(1671):20140152. doi: 10.1098/rstb.2014.0152. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 25964461 Free PMC article. Review.
-
NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles.J Antimicrob Chemother. 2015;70(6):1674-85. doi: 10.1093/jac/dkv034. Epub 2015 Feb 19. J Antimicrob Chemother. 2015. PMID: 25700718 Free PMC article.
-
Nanobody-mediated complement activation to kill HIV-infected cells.EMBO Mol Med. 2023 Apr 11;15(4):e16422. doi: 10.15252/emmm.202216422. Epub 2023 Feb 17. EMBO Mol Med. 2023. PMID: 36799046 Free PMC article.
-
Sensitivity to Broadly Neutralizing Antibodies of Recently Transmitted HIV-1 Clade CRF02_AG Viruses with a Focus on Evolution over Time.J Virol. 2019 Jan 4;93(2):e01492-18. doi: 10.1128/JVI.01492-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30404804 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

