Chondrocyte-specific inhibition of β-catenin signaling leads to dysplasia of the caudal vertebrae in mice
- PMID: 24026150
- PMCID: PMC3928445
- DOI: 10.1097/01.brs.0000435024.57940.8d
Chondrocyte-specific inhibition of β-catenin signaling leads to dysplasia of the caudal vertebrae in mice
Abstract
Study design: To inhibit β-catenin specifically signaling in chondrocytes Col2-ICAT transgenic mice were generated. Anomalies in caudal vertebrae were detected during embryonic and postnatal stages of Col2-ICAT transgenic mice.
Objective: To determine the role of canonical β-catenin signaling in caudal vertebral development.
Summary of background data: β-catenin signaling plays a critical role in skeletal development. Col2-ICAT transgenic mice were generated to selectively block β-catenin signaling by overexpression of the ICAT gene in chondrocytes.
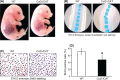
Methods: Tails of E16.5 transgenic embryos and adult Col2-ICAT transgenic mice and their wild-type littermates were collected and analyzed. Skeletal preparation, 3-dimensional micro-computed tomographic and histological analyses were performed to evaluate changes in the structure of caudal vertebrae. Bromodeoxyuridine labeling was performed to evaluate changes in chondrocyte proliferation in caudal vertebrae.
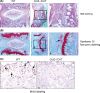
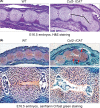
Results: Skeletal preparation and 3-dimensional micro-computed tomographic analyses revealed bone deformation and angulated deformities in tail tissue in Col2-ICAT transgenic mice. Histological studies revealed abnormal bone development and dysplastic caudal vertebrae in Col2-ICAT transgenic mice. Inhibition of β-catenin signaling in cartilage resulted in vertebral dysplasia leading to aberrant resegmenting process. Thus, 2 poorly developed sclerotomes failed to fuse to form a complete vertebrae. BrdU labeling revealed a decreased chondrocyte proliferation in both cartilageous templates of transgenic embryos and the growth plate of adult Col2-ICAT transgenic mice.
Conclusion: Wnt/β-catenin signaling plays an important role in vertebral development. Inhibition of β-catenin signaling in chondrocytes results in caudal vertebra deformity in mice, which may occur as early as in the stage of sclerotome formation.
Level of evidence: N/A.
Figures

Similar articles
-
Inhibition of beta-catenin signaling causes defects in postnatal cartilage development.J Cell Sci. 2008 May 1;121(Pt 9):1455-65. doi: 10.1242/jcs.020362. Epub 2008 Apr 8. J Cell Sci. 2008. PMID: 18397998 Free PMC article.
-
Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction.Arthritis Rheum. 2008 Jul;58(7):2053-64. doi: 10.1002/art.23614. Arthritis Rheum. 2008. PMID: 18576323 Free PMC article.
-
Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice.Am J Pathol. 2009 Nov;175(5):1993-2003. doi: 10.2353/ajpath.2009.081173. Epub 2009 Oct 8. Am J Pathol. 2009. PMID: 19815716 Free PMC article.
-
Beta-catenin, cartilage, and osteoarthritis.Ann N Y Acad Sci. 2010 Mar;1192(1):344-50. doi: 10.1111/j.1749-6632.2009.05212.x. Ann N Y Acad Sci. 2010. PMID: 20392258 Free PMC article. Review.
-
How to build an inducible cartilage-specific transgenic mouse.Arthritis Res Ther. 2014;16(3):210. doi: 10.1186/ar4573. Arthritis Res Ther. 2014. PMID: 25166474 Free PMC article. Review.
Cited by
-
CHIP regulates skeletal development and postnatal bone growth.J Cell Physiol. 2020 Jun;235(6):5378-5385. doi: 10.1002/jcp.29424. Epub 2020 Jan 3. J Cell Physiol. 2020. PMID: 31898815 Free PMC article.
-
TGF-β Initiates β-Catenin-Mediated CTGF Secretory Pathway in Old Bovine Nucleus Pulposus Cells: A Potential Mechanism for Intervertebral Disc Degeneration.JBMR Plus. 2018 Jul 10;3(2):e10069. doi: 10.1002/jbm4.10069. eCollection 2019 Feb. JBMR Plus. 2018. PMID: 30828686 Free PMC article.
References
-
- Flint OP, Ede DA, Wilby OK, et al. Control of somite number in normal and amputated mouse embryos: an experimental and a theoretical analysis. J Embryol Exp Morph 1978;45:189–202 - PubMed
-
- Tam PPL. The control of somitogenesis in mouse embryos. J Embryol Exp Morph 1981;65:103–28 - PubMed
-
- Erlebacher A, Filvaroff EH, Gitelman SE, et al. Toward a molecular understanding of skeletal development. Cell 1995;80:371–8 - PubMed
-
- Karsenty G. Genetics of skeletogenesis. Dev Genet 1998;22:301–13 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

