Design and implementation of a custom built optical projection tomography system
- PMID: 24023880
- PMCID: PMC3762719
- DOI: 10.1371/journal.pone.0073491
Design and implementation of a custom built optical projection tomography system
Abstract
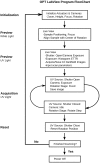
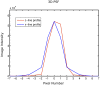
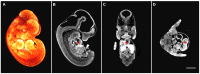
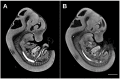
Optical projection tomography (OPT) is an imaging modality that has, in the last decade, answered numerous biological questions owing to its ability to view gene expression in 3 dimensions (3D) at high resolution for samples up to several cm(3). This has increased demand for a cabinet OPT system, especially for mouse embryo phenotyping, for which OPT was primarily designed for. The Medical Research Council (MRC) Technology group (UK) released a commercial OPT system, constructed by Skyscan, called the Bioptonics OPT 3001 scanner that was installed in a limited number of locations. The Bioptonics system has been discontinued and currently there is no commercial OPT system available. Therefore, a few research institutions have built their own OPT system, choosing parts and a design specific to their biological applications. Some of these custom built OPT systems are preferred over the commercial Bioptonics system, as they provide improved performance based on stable translation and rotation stages and up to date CCD cameras coupled with objective lenses of high numerical aperture, increasing the resolution of the images. Here, we present a detailed description of a custom built OPT system that is robust and easy to build and install. Included is a hardware parts list, instructions for assembly, a description of the acquisition software and a free download site, and methods for calibration. The described OPT system can acquire a full 3D data set in 10 minutes at 6.7 micron isotropic resolution. The presented guide will hopefully increase adoption of OPT throughout the research community, for the OPT system described can be implemented by personnel with minimal expertise in optics or engineering who have access to a machine shop.
Conflict of interest statement
Figures


Similar articles
-
OPTiM: Optical projection tomography integrated microscope using open-source hardware and software.PLoS One. 2017 Jul 11;12(7):e0180309. doi: 10.1371/journal.pone.0180309. eCollection 2017. PLoS One. 2017. PMID: 28700724 Free PMC article.
-
Comparison of optical projection tomography and light-sheet fluorescence microscopy.J Microsc. 2019 Jul;275(1):3-10. doi: 10.1111/jmi.12796. Epub 2019 Apr 30. J Microsc. 2019. PMID: 31012490
-
Imaging using parallel integrals in optical projection tomography.Phys Med Biol. 2006 Dec 7;51(23):6023-32. doi: 10.1088/0031-9155/51/23/005. Epub 2006 Oct 30. Phys Med Biol. 2006. PMID: 17110767
-
Optical projection tomography.Annu Rev Biomed Eng. 2004;6:209-28. doi: 10.1146/annurev.bioeng.6.040803.140210. Annu Rev Biomed Eng. 2004. PMID: 15255768 Review.
-
In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. PMID: 26844322 Free Books & Documents. Review.
Cited by
-
Mouse embryo phenotyping using X-ray microCT.Front Cell Dev Biol. 2022 Sep 16;10:949184. doi: 10.3389/fcell.2022.949184. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36187491 Free PMC article. Review.
-
An optimized workflow for microCT imaging of formalin-fixed and paraffin-embedded (FFPE) early equine embryos.Anat Histol Embryol. 2022 Sep;51(5):611-623. doi: 10.1111/ahe.12834. Epub 2022 Jul 18. Anat Histol Embryol. 2022. PMID: 35851500 Free PMC article.
-
3D imaging of human organs with micrometer resolution - applied to the endocrine pancreas.Commun Biol. 2021 Sep 10;4(1):1063. doi: 10.1038/s42003-021-02589-x. Commun Biol. 2021. PMID: 34508173 Free PMC article.
-
Survey of spiking in the mouse visual system reveals functional hierarchy.Nature. 2021 Apr;592(7852):86-92. doi: 10.1038/s41586-020-03171-x. Epub 2021 Jan 20. Nature. 2021. PMID: 33473216 Free PMC article.
-
Mesoscopic 3D imaging of pancreatic cancer and Langerhans islets based on tissue autofluorescence.Sci Rep. 2020 Oct 26;10(1):18246. doi: 10.1038/s41598-020-74616-6. Sci Rep. 2020. PMID: 33106532 Free PMC article.
References
-
- Ellegood J, Lerch JP, Henkelman RM (2011) Brain abnormalities in a Neuroligin3 R451C knockin mouse model associated with autism. Autism Res 4: 368–376 doi:10.1002/aur.215 - DOI - PubMed
-
- Lerch JP, Carroll JB, Spring S, Bertram LN, Schwab C, et al. (2008) Automated deformation analysis in the YAC128 Huntington disease mouse model. NeuroImage 39: 32–39 doi:10.1016/j.neuroimage.2007.08.033 - DOI - PubMed
-
- Schneider JE, Bamforth SD, Farthing CR, Clarke K, Neubauer S, et al. (2003) Rapid identification and 3D reconstruction of complex cardiac malformations in transgenic mouse embryos using fast gradient echo sequence magnetic resonance imaging. J Mol Cell Cardiol 35: 217–222. - PubMed
-
- Cleary JO, Price AN, Thomas DL, Scambler PJ, Kyriakopoulou V, et al. (2009) Cardiac phenotyping in ex vivo murine embryos using microMRI. NMR Biomed 22: 857–866 doi:10.1002/nbm.1400 - DOI - PubMed
-
- Nieman BJ, Flenniken AM, Adamson SL, Henkelman RM, Sled JG (2006) Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiological Genomics 24: 154–162 doi:10.1152/physiolgenomics.00217.2005 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

