Development of a culture system to induce microglia-like cells from haematopoietic cells
- PMID: 24016036
- PMCID: PMC4282385
- DOI: 10.1111/nan.12086
Development of a culture system to induce microglia-like cells from haematopoietic cells
Abstract
Aims: Microglia are the resident immune cells in the central nervous system, originating from haematopoietic-derived myeloid cells. A microglial cell is a double-edged sword, which has both pro-inflammatory and anti-inflammatory functions. Although understanding the role of microglia in pathological conditions has become increasingly important, histopathology has been the only way to investigate microglia in human diseases.
Methods: To enable the study of microglial cells in vitro, we here establish a culture system to induce microglia-like cells from haematopoietic cells by coculture with astrocytes. The characteristics of microglia-like cells were analysed by flow cytometry and functional assay.
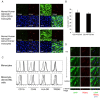
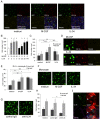
Results: We show that triggering receptor expressing on myeloid cells-2-expressing microglia-like cells could be induced from lineage negative cells or monocytes by coculture with astrocytes. Microglia-like cells exhibited lower expression of CD45 and MHC class II than macrophages, a characteristic similar to brain microglia. When introduced into brain slice cultures, these microglia-like cells changed their morphology to a ramified shape on the first day of the culture. Moreover, we demonstrated that microglia-like cells could be induced from human monocytes by coculture with astrocytes. Finally, we showed that interleukin 34 was an important factor in the induction of microglia-like cells from haematopoietic cells in addition to cell-cell contact with astrocytes. Purified microglia-like cells were suitable for further culture and functional analyses.
Conclusion: Development of in vitro induction system for microglia will further promote the study of human microglial cells under pathological conditions as well as aid in the screening of drugs to target microglial cells.
Keywords: astrocytes; haematopoietic cells; interleukin 34; microglia; monocytes; triggering receptor expressing on myeloid cells-2 (TREM2).
© 2013 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of the British Neuropathological Society.
Figures
Similar articles
-
Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: morphology.Glia. 1994 Dec;12(4):245-58. doi: 10.1002/glia.440120402. Glia. 1994. PMID: 7890329
-
Ramification of microglia, monocytes and macrophages in vitro: influences of various epithelial and mesenchymal cells and their conditioned media.Cell Tissue Res. 1997 Feb;287(3):447-58. doi: 10.1007/s004410050769. Cell Tissue Res. 1997. PMID: 9023076
-
In vitro differentiation of lineage-negative bone marrow cells into microglia-like cells.Eur J Neurosci. 2010 Apr;31(7):1155-63. doi: 10.1111/j.1460-9568.2010.07152.x. Epub 2010 Mar 19. Eur J Neurosci. 2010. PMID: 20345919
-
Cellular players that shape evolving pathology and neurodegeneration following traumatic brain injury.Brain Behav Immun. 2018 Jul;71:9-17. doi: 10.1016/j.bbi.2018.03.033. Epub 2018 Mar 27. Brain Behav Immun. 2018. PMID: 29601944 Review.
-
Microglial cells in astroglial cultures: a cautionary note.J Neuroinflammation. 2007 Oct 15;4:26. doi: 10.1186/1742-2094-4-26. J Neuroinflammation. 2007. PMID: 17937799 Free PMC article. Review.
Cited by
-
Suicide and Microglia: Recent Findings and Future Perspectives Based on Human Studies.Front Cell Neurosci. 2019 Feb 13;13:31. doi: 10.3389/fncel.2019.00031. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30814929 Free PMC article.
-
A human microglia-like cellular model for assessing the effects of neurodegenerative disease gene variants.Sci Transl Med. 2017 Dec 20;9(421):eaai7635. doi: 10.1126/scitranslmed.aai7635. Sci Transl Med. 2017. PMID: 29263232 Free PMC article.
-
Patient-Derived In Vitro Models of Microglial Function and Synaptic Engulfment in Schizophrenia.Biol Psychiatry. 2022 Sep 15;92(6):470-479. doi: 10.1016/j.biopsych.2022.01.004. Epub 2022 Jan 19. Biol Psychiatry. 2022. PMID: 35232567 Free PMC article. Review.
-
The adenosine generating enzymes CD39/CD73 control microglial processes ramification in the mouse brain.PLoS One. 2017 Apr 4;12(4):e0175012. doi: 10.1371/journal.pone.0175012. eCollection 2017. PLoS One. 2017. PMID: 28376099 Free PMC article.
-
Human microglial models to study HIV infection and neuropathogenesis: a literature overview and comparative analyses.J Neurovirol. 2022 Feb;28(1):64-91. doi: 10.1007/s13365-021-01049-w. Epub 2022 Feb 9. J Neurovirol. 2022. PMID: 35138593 Free PMC article. Review.
References
-
- Akiyama H, McGeer PL. Specificity of mechanisms for plaque removal after A beta immunotherapy for Alzheimer disease. Nat Med. 2004;10:117–118. - PubMed
-
- Sanders P, De Keyser J. Janus faces of microglia in multiple sclerosis. Brain Res Rev. 2007;54:274–285. - PubMed
-
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75:388–397. - PubMed
-
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

