The extracellular protein VlsE is destabilized inside cells
- PMID: 24013077
- PMCID: PMC4059009
- DOI: 10.1016/j.jmb.2013.08.024
The extracellular protein VlsE is destabilized inside cells
Abstract
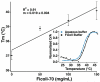
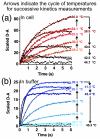
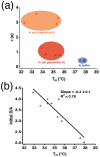
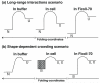
We use U2OS cells as in vivo "test tubes" to study how the same cytoplasmic environment has opposite effects on the stability of two different proteins. Protein folding stability and kinetics were compared by fast relaxation imaging, which combines a temperature jump with fluorescence microscopy of FRET (Förster resonance energy transfer)-labeled proteins. While the stability of the cytoplasmic enzyme PGK (phosphoglycerate kinase) increases in cells, the stability of the cell surface antigen VlsE, which presumably did not evolve for stability inside cells, decreases. VlsE folding also slows down more than PGK folding in cells, relative to their respective aqueous buffer kinetics. Our FRET measurements provide evidence that VlsE is more compact inside cells than in aqueous buffer. Two kinetically distinct protein populations exist inside cells, making a connection with previous in vitro crowding studies. In addition, we confirm previous studies showing that VlsE is stabilized by 150mg/mL of the carbohydrate crowder Ficoll, even though it is destabilized in the cytoplasm relative to aqueous buffer. We propose two mechanisms for the observed destabilization of VlsE in U2OS cells: long-range interactions competing with crowding or shape-dependent crowding favoring more compact states inside the cell over the elongated aqueous buffer native state.
Keywords: extracellular protein; fast relaxation imaging (FReI); fluorescence resonance energy transfer (FRET); protein folding; variable major protein-like sequence expressed (VlsE).
© 2013.
Figures


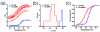
Similar articles
-
An in vitro mimic of in-cell solvation for protein folding studies.Protein Sci. 2020 Apr;29(4):1060-1068. doi: 10.1002/pro.3833. Epub 2020 Feb 6. Protein Sci. 2020. PMID: 31994240 Free PMC article.
-
Non-Steric Interactions Predict the Trend and Steric Interactions the Offset of Protein Stability in Cells.Chemphyschem. 2018 Sep 18;19(18):2290-2294. doi: 10.1002/cphc.201800534. Epub 2018 Jun 21. Chemphyschem. 2018. PMID: 29877016
-
Subcellular modulation of protein VlsE stability and folding kinetics.FEBS Lett. 2016 May;590(10):1409-16. doi: 10.1002/1873-3468.12193. Epub 2016 May 17. FEBS Lett. 2016. PMID: 27129718
-
Folding, stability and shape of proteins in crowded environments: experimental and computational approaches.Int J Mol Sci. 2009 Feb;10(2):572-588. doi: 10.3390/ijms10020572. Epub 2009 Feb 13. Int J Mol Sci. 2009. PMID: 19333422 Free PMC article. Review.
-
Simulating protein folding in different environmental conditions.Adv Exp Med Biol. 2014;805:171-97. doi: 10.1007/978-3-319-02970-2_8. Adv Exp Med Biol. 2014. PMID: 24446362 Review.
Cited by
-
Exploring the Denatured State Ensemble by Single-Molecule Chemo-Mechanical Unfolding: The Effect of Force, Temperature, and Urea.J Mol Biol. 2018 Feb 16;430(4):450-464. doi: 10.1016/j.jmb.2017.07.022. Epub 2017 Aug 4. J Mol Biol. 2018. PMID: 28782558 Free PMC article.
-
An in vitro mimic of in-cell solvation for protein folding studies.Protein Sci. 2020 Apr;29(4):1060-1068. doi: 10.1002/pro.3833. Epub 2020 Feb 6. Protein Sci. 2020. PMID: 31994240 Free PMC article.
-
Environmental Fluctuations and Stochastic Resonance in Protein Folding.Chemphyschem. 2016 May 4;17(9):1341-8. doi: 10.1002/cphc.201501041. Epub 2016 Jan 20. Chemphyschem. 2016. PMID: 26711088 Free PMC article.
-
Excluded-volume effects in living cells.Angew Chem Int Ed Engl. 2015 Feb 16;54(8):2548-51. doi: 10.1002/anie.201409847. Epub 2014 Dec 29. Angew Chem Int Ed Engl. 2015. PMID: 25557778 Free PMC article.
-
In-cell destabilization of a homodimeric protein complex detected by DEER spectroscopy.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20566-20575. doi: 10.1073/pnas.2005779117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788347 Free PMC article.
References
-
- Dobson CM. Principles of protein folding, misfolding and aggregation. Seminars in Cell & Developmental Biology. 2004;15:3–16. - PubMed
-
- Ellis RJ. Molecular chaperones: avoiding the crowd. Current Biology. 1997;7:R531–R533. - PubMed
-
- Agashe VR, Hartl FU. Roles of molecular chaperones in cytoplasmic protein folding. Seminars in Cell and Developmental Biology. 2000;11:15–25. - PubMed
-
- Ebbinghaus S, Dhar A, McDonald JD, Gruebele M. Protein folding stability and dynamics imaged in a living cell. Nature Methods. 2010;7:319–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

