Tumor-targeting TRAIL expression mediated by miRNA response elements suppressed growth of uveal melanoma cells
- PMID: 24001901
- PMCID: PMC5528445
- DOI: 10.1016/j.molonc.2013.08.003
Tumor-targeting TRAIL expression mediated by miRNA response elements suppressed growth of uveal melanoma cells
Abstract
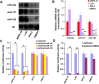
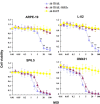
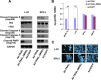
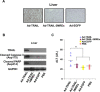
Malignant uveal melanoma severely damages eye function and is prone to metastasize to other organs. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising agent to treat uveal melanoma because of its induction of apoptosis in cancer cells both at primary and metastatic sites. However, TRAIL therapy lacks tumor specificity in the current delivery systems for uveal melanoma treatment, thereby causing cytotoxiciy to normal tissues. To improve uveal melanoma specificity of adenovirus-based TRAIL introduction, we used miRNA response elements (MREs) of miR-34a, miR-137 and miR-182, which have been shown to have reduced expression in uveal melanoma cells, to regulate its expression. miR-34a, miR-137 and miR-182 all had lower expression levels in uveal melanoma cell lines, compared with normal cells. MREs-regulated luciferase activity was reduced in normal cell lines, but not significantly attenuated in uveal melanoma cells. The infection of MRE-regulated TRAIL-expressing adenoviral vector (Ad-TRAIL-3MREs) led to high level of TRAIL expression in uveal melanoma cell lines, but not in normal cells. Strong expression of TRAIL had a high anti-tumor capacity by inducing apoptosis in uveal melanoma cells. In contrast, Ad-TRAIL-3MREs had no cytotoxicity to normal cell lines. Animal experiments further confirmed tumor-suppressing effect of Ad-TRAIL-3MREs on uveal melanoma xenografts and its biosafety to hepatic tissues. Collectively, we constructed an MRE-directed TRAIL-expressing adenoviral vector and provided evidence that this vector possessed high anti-tumor activity and uveal melanoma specificity.
Keywords: Adenovirus; TRAIL; Uveal melanoma; miRNA.
Crown Copyright © 2013. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
MiRNA regulation of TRAIL expression exerts selective cytotoxicity to prostate carcinoma cells.Mol Cell Biochem. 2014 Mar;388(1-2):123-33. doi: 10.1007/s11010-013-1904-3. Epub 2013 Nov 30. Mol Cell Biochem. 2014. PMID: 24292881
-
microRNA response elements-regulated TRAIL expression shows specific survival-suppressing activity on bladder cancer.J Exp Clin Cancer Res. 2013 Feb 26;32(1):10. doi: 10.1186/1756-9966-32-10. J Exp Clin Cancer Res. 2013. PMID: 23442927 Free PMC article.
-
Selective TRAIL-induced cytotoxicity to lung cancer cells mediated by miRNA response elements.Cell Biochem Funct. 2014 Oct;32(7):547-56. doi: 10.1002/cbf.3042. Epub 2014 Aug 1. Cell Biochem Funct. 2014. PMID: 25132116
-
Oncogenic signaling in uveal melanoma.Pigment Cell Melanoma Res. 2018 Nov;31(6):661-672. doi: 10.1111/pcmr.12708. Epub 2018 May 23. Pigment Cell Melanoma Res. 2018. PMID: 29738114 Review.
-
Metastatic uveal melanoma: biology and emerging treatments.Cancer J. 2012 Mar-Apr;18(2):148-52. doi: 10.1097/PPO.0b013e31824bd256. Cancer J. 2012. PMID: 22453016 Free PMC article. Review.
Cited by
-
MicroRNAs and Uveal Melanoma: Understanding the Diverse Role of These Small Molecular Regulators.Int J Mol Sci. 2020 Aug 6;21(16):5648. doi: 10.3390/ijms21165648. Int J Mol Sci. 2020. PMID: 32781746 Free PMC article. Review.
-
miR-26b Mimic Inhibits Glioma Proliferation In Vitro and In Vivo Suppressing COX-2 Expression.Oncol Res. 2019 Feb 5;27(2):147-155. doi: 10.3727/096504017X15021536183517. Epub 2017 Aug 11. Oncol Res. 2019. PMID: 28800785 Free PMC article.
-
Publication trends of research on uveal melanoma during 2000-2020: a 20-year bibliometric study.Ann Transl Med. 2020 Nov;8(21):1463. doi: 10.21037/atm-20-3700. Ann Transl Med. 2020. PMID: 33313208 Free PMC article. Review.
-
Emerging roles of microRNAs and their implications in uveal melanoma.Cell Mol Life Sci. 2021 Jan;78(2):545-559. doi: 10.1007/s00018-020-03612-w. Epub 2020 Aug 11. Cell Mol Life Sci. 2021. PMID: 32783068 Free PMC article. Review.
-
TNF-related apoptosis inducing ligand in ocular cancers and ocular diabetic complications.Biomed Res Int. 2015;2015:424019. doi: 10.1155/2015/424019. Epub 2015 Mar 5. Biomed Res Int. 2015. PMID: 25834817 Free PMC article. Review.
References
-
- Alizadeh, H. , Howard, K. , Mellon, J. , Mayhew, E. , Rusciano, D. , Niederkorn, J.Y. , 2003. Reduction of liver metastasis of intraocular melanoma by interferon-beta gene transfer. Invest. Ophthalmol. Vis. Sci.. 44, 3042–3051. - PubMed
-
- Armeanu, S. , Lauer, U.M. , Smirnow, I. , Schenk, M. , Weiss, T.S. , Gregor, M. , Bitzer, M. , 2003. Adenoviral gene transfer of tumor necrosis factor-related apoptosis-inducing ligand overcomes an impaired response of hepatoma cells but causes severe apoptosis in primary human hepatocytes. Cancer Res.. 63, 2369–2372. - PubMed
-
- Bakalian, S. , Marshall, J.C. , Logan, P. , Faingold, D. , Maloney, S. , Di Cesare, S. , Martins, C. , Fernandes, B.F. , Burnier, M.N. , 2008. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin. Cancer Res.. 14, 951–956. - PubMed
-
- Bo, Y. , Guo, G. , Yao, W. , 2013. MiRNA-mediated tumor specific delivery of TRAIL reduced glioma growth. J. Neurooncol.. 112, 27–37. - PubMed
-
- Carlo-Stella, C. , Lavazza, C. , Locatelli, A. , Vigano, L. , Gianni, A.M. , Gianni, L. , 2007. Targeting TRAIL agonistic receptors for cancer therapy. Clin. Cancer Res.. 13, 2313–2317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

