Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells
- PMID: 23995070
- PMCID: PMC4106025
- DOI: 10.1038/nn.3505
Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells
Abstract
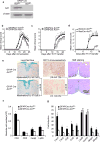
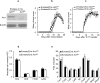
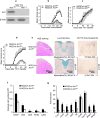
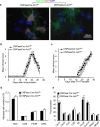
Interleukin 17 (IL-17) is a signature cytokine of Th17 cells. We previously reported that deletion of NF-κB activator 1 (Act1), the key transducer of IL-17 receptor signaling, from the neuroectodermal lineage in mice (neurons, oligodendrocytes and astrocytes) results in attenuated severity of experimental autoimmune encephalomyelitis (EAE). Here we examined the cellular basis of this observation. EAE disease course was unaffected by deletion of Act1 in neurons or mature oligodendrocytes, and Act1 deletion in astrocytes only modestly affected disease course. Deletion of Act1 in NG2(+) glia resulted in markedly reduced EAE severity. Furthermore, IL-17 induced characteristic inflammatory mediator expression in NG2(+) glial cells. IL-17 also exhibited strong inhibitory effects on the maturation of oligodendrocyte lineage cells in vitro and reduced their survival. These data identify NG2(+) glia as the major CNS cellular target of IL-17 in EAE. The sensitivity of oligodendrocyte lineage cells to IL-17-mediated toxicity further suggests a direct link between inflammation and neurodegeneration in multiple sclerosis.
Figures
Similar articles
-
Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis.Immunity. 2010 Mar 26;32(3):414-25. doi: 10.1016/j.immuni.2010.03.004. Epub 2010 Mar 18. Immunity. 2010. PMID: 20303295 Free PMC article.
-
Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis.J Neuroinflammation. 2009 Apr 28;6:14. doi: 10.1186/1742-2094-6-14. J Neuroinflammation. 2009. PMID: 19400960 Free PMC article.
-
CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes.Mol Ther. 2012 Jul;20(7):1338-48. doi: 10.1038/mt.2012.12. Epub 2012 Mar 20. Mol Ther. 2012. PMID: 22434134 Free PMC article.
-
IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease.Trends Immunol. 2011 May;32(5):232-9. doi: 10.1016/j.it.2011.02.007. Epub 2011 Apr 12. Trends Immunol. 2011. PMID: 21493143 Free PMC article. Review.
-
Function of Act1 in IL-17 family signaling and autoimmunity.Adv Exp Med Biol. 2012;946:223-35. doi: 10.1007/978-1-4614-0106-3_13. Adv Exp Med Biol. 2012. PMID: 21948371 Free PMC article. Review.
Cited by
-
TRAF3IP2 mediates aldosterone/salt-induced cardiac hypertrophy and fibrosis.Mol Cell Endocrinol. 2016 Jul 5;429:84-92. doi: 10.1016/j.mce.2016.03.038. Epub 2016 Apr 1. Mol Cell Endocrinol. 2016. PMID: 27040306 Free PMC article.
-
Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF).Biomaterials. 2015 Jul;56:78-85. doi: 10.1016/j.biomaterials.2015.03.044. Epub 2015 Apr 15. Biomaterials. 2015. PMID: 25934281 Free PMC article.
-
Violacein Treatment Modulates Acute and Chronic Inflammation through the Suppression of Cytokine Production and Induction of Regulatory T Cells.PLoS One. 2015 May 4;10(5):e0125409. doi: 10.1371/journal.pone.0125409. eCollection 2015. PLoS One. 2015. PMID: 25938431 Free PMC article.
-
Interleukin-17 in Chronic Inflammatory Neurological Diseases.Front Immunol. 2020 Jun 3;11:947. doi: 10.3389/fimmu.2020.00947. eCollection 2020. Front Immunol. 2020. PMID: 32582147 Free PMC article. Review.
-
TH Cells and Cytokines in Encephalitogenic Disorders.Front Immunol. 2022 Mar 7;13:822919. doi: 10.3389/fimmu.2022.822919. eCollection 2022. Front Immunol. 2022. PMID: 35320935 Free PMC article. Review.
References
-
- Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med (Berl) 2006;84:532–543. - PubMed
-
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. - PubMed
-
- Steinman L. Multiple sclerosis: a two-stage disease. Nat Immunol. 2001;2:762–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

