Sost and its paralog Sostdc1 coordinate digit number in a Gli3-dependent manner
- PMID: 23994639
- PMCID: PMC3861057
- DOI: 10.1016/j.ydbio.2013.08.015
Sost and its paralog Sostdc1 coordinate digit number in a Gli3-dependent manner
Abstract
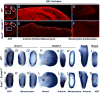
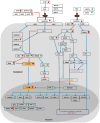
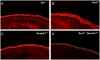
WNT signaling is critical in most aspects of skeletal development and homeostasis, and antagonists of WNT signaling are emerging as key regulatory proteins with great promise as therapeutic agents for bone disorders. Here we show that Sost and its paralog Sostdc1 emerged through ancestral genome duplication and their expression patterns have diverged to delineate non-overlapping domains in most organ systems including musculoskeletal, cardiovascular, nervous, digestive, reproductive and respiratory. In the developing limb, Sost and Sostdc1 display dynamic expression patterns with Sost being restricted to the distal ectoderm and Sostdc1 to the proximal ectoderm and the mesenchyme. While Sostdc1(-/-) mice lack any obvious limb or skeletal defects, Sost(-/-) mice recapitulate the hand defects described for Sclerosteosis patients. However, elevated WNT signaling in Sost(-/-); Sostdc1(-/-) mice causes misregulation of SHH signaling, ectopic activation of Sox9 in the digit 1 field and preaxial polydactyly in a Gli1- and Gli3-dependent manner. In addition, we show that the syndactyly documented in Sclerosteosis is present in both Sost(-/-) and Sost(-/-); Sostdc1(-/-) mice, and is driven by misregulation of Fgf8 in the AER, a region lacking Sost and Sostdc1 expression. This study highlights the complexity of WNT signaling in skeletal biology and disease and emphasizes how redundant mechanism and non-cell autonomous effects can synergize to unveil new intricate phenotypes caused by elevated WNT signaling.
Keywords: Limb formation; Polydactyly; Sclerostin; Shh; Sost; Sostdc1; WNT signaling; syndactyly.
© 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon.Dev Biol. 2003 Aug 15;260(2):484-95. doi: 10.1016/s0012-1606(03)00252-5. Dev Biol. 2003. PMID: 12921747
-
Limb anterior-posterior polarity integrates activator and repressor functions of GLI2 as well as GLI3.Dev Biol. 2012 Oct 1;370(1):110-24. doi: 10.1016/j.ydbio.2012.07.017. Epub 2012 Jul 25. Dev Biol. 2012. PMID: 22841643 Free PMC article.
-
Genetic evidence that SOST inhibits WNT signaling in the limb.Dev Biol. 2010 Jun 15;342(2):169-79. doi: 10.1016/j.ydbio.2010.03.021. Epub 2010 Mar 30. Dev Biol. 2010. PMID: 20359476 Free PMC article.
-
Wnt Antagonists in Hematopoietic and Immune Cell Fate: Implications for Osteoporosis Therapies.Curr Osteoporos Rep. 2019 Apr;17(2):49-58. doi: 10.1007/s11914-019-00503-3. Curr Osteoporos Rep. 2019. PMID: 30835038 Free PMC article. Review.
-
Genetics of Sost/SOST in sclerosteosis and van Buchem disease animal models.Metabolism. 2018 Mar;80:38-47. doi: 10.1016/j.metabol.2017.10.005. Epub 2017 Oct 25. Metabolism. 2018. PMID: 29080811 Review.
Cited by
-
Lrp4 in osteoblasts suppresses bone formation and promotes osteoclastogenesis and bone resorption.Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):3487-92. doi: 10.1073/pnas.1419714112. Epub 2015 Mar 2. Proc Natl Acad Sci U S A. 2015. PMID: 25733894 Free PMC article.
-
Sclerostin expression and functions beyond the osteocyte.Bone. 2017 Mar;96:45-50. doi: 10.1016/j.bone.2016.11.024. Epub 2016 Nov 23. Bone. 2017. PMID: 27888056 Free PMC article. Review.
-
Single-Cell Transcriptomics Reveals that Differentiation and Spatial Signatures Shape Epidermal and Hair Follicle Heterogeneity.Cell Syst. 2016 Sep 28;3(3):221-237.e9. doi: 10.1016/j.cels.2016.08.010. Epub 2016 Sep 15. Cell Syst. 2016. PMID: 27641957 Free PMC article.
-
Sclerostin: From Molecule to Clinical Biomarker.Int J Mol Sci. 2022 Apr 26;23(9):4751. doi: 10.3390/ijms23094751. Int J Mol Sci. 2022. PMID: 35563144 Free PMC article. Review.
-
Niches for Skeletal Stem Cells of Mesenchymal Origin.Front Cell Dev Biol. 2020 Jul 10;8:592. doi: 10.3389/fcell.2020.00592. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32754592 Free PMC article. Review.
References
-
- Akiyama H, Stadler HS, Martin JF, Ishii TM, Beachy PA, Nakamura T, de Crombrugghe B. Misexpression of Sox9 in mouse limb bud mesenchyme induces polydactyly and rescues hypodactyly mice. Matrix Biol: J Int Soc Matrix Biol. 2007;26:224–233. - PubMed
-
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

