Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling
- PMID: 23993924
- PMCID: PMC4266321
- DOI: 10.1016/j.fertnstert.2013.07.1978
Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling
Abstract
Objective: To test whether bone morphogenetic protein 4 (BMP4) directly regulates differentiation of adult mouse ovary-derived oogonial stem cells (OSCs) in vitro.
Design: Animal study.
Setting: Research laboratory.
Animal(s): Adult C57BL/6 female mice.
Intervention(s): After purification from adult ovaries by fluorescence-activated cell sorting, OSCs were cultured without or with BMP4 in the absence or presence of the BMP4 antagonist, Noggin.
Main outcome measure(s): Rates of in vitro-derived (IVD) oocyte formation and changes in gene expression were assessed.
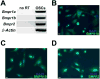
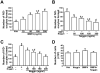
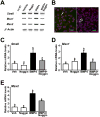
Result(s): Cultured OSCs expressed BMP receptor (BMPR) 1A (BMPR1A), BMPR1B, and BMPR2, suggesting that BMP signaling can directly affect OSC function. In agreement with this, BMP4 significantly increased the number of IVD oocytes formed by cultured OSCs in a dose-dependent manner, and this response was inhibited in a dose-dependent fashion by cotreatment with Noggin. Exposure of OSCs to BMP4 was associated with rapid phosphorylation of BMPR-regulated Smad1/5/8 proteins, and this response was followed by increased expression of the meiosis initiation factors, stimulated by retinoic acid gene 8 (Stra8), muscle-segment homeobox 1 (Msx1), and Msx2. In keeping with the IVD oocyte formation data, the ability of BMP4 to activate Smad1/5/8 signaling and meiotic gene expression in OSCs was abolished by cotreatment with Noggin.
Conclusion(s): Engagement of BMP4-mediated signaling in adult mouse ovary-derived OSCs cultured in vitro drives differentiation of these cells into IVD oocytes through Smad1/5/8 activation and transcriptional up-regulation of key meiosis-initiating genes.
Keywords: BMP4; germ cells; oocyte; oogenesis; stem cells.
Copyright © 2013 American Society for Reproductive Medicine. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity.Fertil Steril. 2013 Nov;100(5):1451-8. doi: 10.1016/j.fertnstert.2013.06.036. Epub 2013 Jul 19. Fertil Steril. 2013. PMID: 23876535 Free PMC article.
-
Fstl1 Promotes Glioma Growth Through the BMP4/Smad1/5/8 Signaling Pathway.Cell Physiol Biochem. 2017;44(4):1616-1628. doi: 10.1159/000485759. Epub 2017 Dec 6. Cell Physiol Biochem. 2017. PMID: 29212066
-
Dynamic expression of bone morphogenetic protein 4 in reproductive organs of female mice.Reproduction. 2011 Oct;142(4):573-9. doi: 10.1530/REP-10-0299. Epub 2011 Aug 2. Reproduction. 2011. PMID: 21810858
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
Implications and Current Limitations of Oogenesis from Female Germline or Oogonial Stem Cells in Adult Mammalian Ovaries.Cells. 2019 Jan 28;8(2):93. doi: 10.3390/cells8020093. Cells. 2019. PMID: 30696098 Free PMC article. Review.
Cited by
-
Comparison of the efficacy of bone morphogenetic protein-4 on in vitro differentiation of murine adipose and bone marrow mesenchymal stem cells into primordial germ cells.Res Pharm Sci. 2022 Jan 15;17(2):123-133. doi: 10.4103/1735-5362.335171. eCollection 2022 Apr. Res Pharm Sci. 2022. PMID: 35280837 Free PMC article.
-
Ovarian germline stem cells.Stem Cell Res Ther. 2014 Aug 18;5(4):98. doi: 10.1186/scrt487. Stem Cell Res Ther. 2014. PMID: 25157949 Free PMC article. Review.
-
Combination therapeutics in complex diseases.J Cell Mol Med. 2016 Dec;20(12):2231-2240. doi: 10.1111/jcmm.12930. Epub 2016 Sep 7. J Cell Mol Med. 2016. PMID: 27605177 Free PMC article. Review.
-
Stem cells, progenitor cells, and lineage decisions in the ovary.Endocr Rev. 2015 Feb;36(1):65-91. doi: 10.1210/er.2014-1079. Epub 2014 Dec 26. Endocr Rev. 2015. PMID: 25541635 Free PMC article. Review.
-
Female Fertility Preservation through Stem Cell-based Ovarian Tissue Reconstitution In Vitro and Ovarian Regeneration In Vivo.Clin Med Insights Reprod Health. 2019 May 23;13:1179558119848007. doi: 10.1177/1179558119848007. eCollection 2019. Clin Med Insights Reprod Health. 2019. PMID: 31191070 Free PMC article. Review.
References
-
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–6. - PubMed
-
- Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, et al. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–70. - PubMed
-
- Zou K, Hou L, Sun K, Xie W, Wu J. Improved efficiency of female germline stem cell purification using Fragilis-based magnetic bead sorting. Stem Cells Dev. 2011;20:2197–204. - PubMed
-
- Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

