Impact of aldosterone antagonists on the substrate for atrial fibrillation: aldosterone promotes oxidative stress and atrial structural/electrical remodeling
- PMID: 23993726
- PMCID: PMC4062912
- DOI: 10.1016/j.ijcard.2013.08.022
Impact of aldosterone antagonists on the substrate for atrial fibrillation: aldosterone promotes oxidative stress and atrial structural/electrical remodeling
Abstract
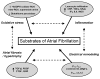
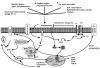
Atrial fibrillation (AF), the most common cardiac arrhythmia, is an electrocardiographic description of a condition with multiple and complex underlying mechanisms. Oxidative stress is an important driver of structural remodeling that creates a substrate for AF. Oxidant radicals may promote increase of atrial oxidative damage, electrical and structural remodeling, and atrial inflammation. AF and other cardiovascular morbidities activate angiotensin (Ang-II)-dependent and independent cascades. A key component of the renin-angiotensin-aldosterone system (RAAS) is the mineralocorticoid aldosterone. Recent studies provide evidence of myocardial aldosterone synthesis. Aldosterone promotes cardiac oxidative stress, inflammation and structural/electrical remodeling via multiple mechanisms. In HF patients, aldosterone production is enhanced. In patients and in experimental HF and AF models, aldosterone receptor antagonists have favorable influences on cardiac remodeling and oxidative stress. Therapeutic approaches that seek to reduce AF burden by modulating the aldosterone system are likely beneficial but underutilized.
Keywords: Aldosterone; Aldosterone antagonist; Atrial fibrillation; Oxidative stress.
© 2013.
Figures
Similar articles
-
Effects of aldosterone and mineralocorticoid receptor antagonism on cardiac ion channels in the view of upstream therapy of atrial fibrillation.Gen Physiol Biophys. 2011 Mar;30(1):11-9. doi: 10.4149/gpb_2011_01_11. Gen Physiol Biophys. 2011. PMID: 21460407 Review.
-
Aldosterone-receptor antagonism as a potential therapeutic option for atrial fibrillation.Br J Pharmacol. 2010 Apr;159(8):1581-3. doi: 10.1111/j.1476-5381.2010.00675.x. Br J Pharmacol. 2010. PMID: 20388187 Free PMC article.
-
Eplerenone Reduces Atrial Fibrillation Burden Without Preventing Atrial Electrical Remodeling.J Am Coll Cardiol. 2017 Dec 12;70(23):2893-2905. doi: 10.1016/j.jacc.2017.10.014. J Am Coll Cardiol. 2017. PMID: 29216985 Free PMC article.
-
Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials.Int J Cardiol. 2013 Apr 30;165(1):17-24. doi: 10.1016/j.ijcard.2012.02.009. Epub 2012 Mar 14. Int J Cardiol. 2013. PMID: 22421406 Review.
-
Antiarrhythmic potential of aldosterone antagonists in atrial fibrillation.Cardiol J. 2012;19(3):223-9. doi: 10.5603/cj.2012.0043. Cardiol J. 2012. PMID: 22641540 Review.
Cited by
-
Atrial Fibrillation and Its Association with Endocrine Disorders.J Atr Fibrillation. 2014 Feb 28;6(5):959. doi: 10.4022/jafib.959. eCollection 2014 Feb-Mar. J Atr Fibrillation. 2014. PMID: 27957035 Free PMC article. Review.
-
Atrial Cardiomyopathy in Valvular Heart Disease: From Molecular Biology to Clinical Perspectives.Cells. 2023 Jul 6;12(13):1796. doi: 10.3390/cells12131796. Cells. 2023. PMID: 37443830 Free PMC article. Review.
-
Atrial fibrillation in heart failure patients: An update on renin-angiotensin-aldosterone system pathway blockade as a therapeutic and prevention target.Cardiol J. 2023;30(2):312-326. doi: 10.5603/CJ.a2022.0061. Epub 2022 Jun 28. Cardiol J. 2023. PMID: 35762070 Free PMC article.
-
Corilagin inhibits angiotensin II-induced atrial fibrosis and fibrillation in mice through the PI3K-Akt pathway.Iran J Basic Med Sci. 2024;27(6):717-724. doi: 10.22038/IJBMS.2024.73281.15928. Iran J Basic Med Sci. 2024. PMID: 38645493 Free PMC article.
-
Oxidant and Inflammatory Mechanisms and Targeted Therapy in Atrial Fibrillation: An Update.J Cardiovasc Pharmacol. 2015 Dec;66(6):523-9. doi: 10.1097/FJC.0000000000000313. J Cardiovasc Pharmacol. 2015. PMID: 26335221 Free PMC article. Review.
References
-
- Goette A, Ittenson A, Hoffmanns P, et al. Increased expression of P-selectin in patients with chronic atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:1872–5. - PubMed
-
- Kozlowski D, Budrejko S, Lip GY, et al. Lone atrial fibrillation: what do we know? Heart. 2009;96:498–503. - PubMed
-
- Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

