Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4⁺ T cell responses and viral control during chronic infection
- PMID: 23993651
- PMCID: PMC4701058
- DOI: 10.1016/j.immuni.2013.08.010
Cell-intrinsic IL-27 and gp130 cytokine receptor signaling regulates virus-specific CD4⁺ T cell responses and viral control during chronic infection
Abstract
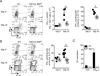
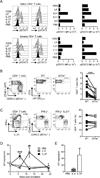
The outcome of chronic viral infections, which affect millions of people worldwide, is greatly dependent on CD4⁺ T cells. Here we showed that T cell-specific ablation of the common interleukin-6 (IL-6) family receptor, gp130, profoundly compromised virus-specific CD4⁺ T cell survival, T follicular helper responses, and IL-21 production at late stages of chronic lymphocytic choriomeningitis virus (LCMV) infection. These effects were cell intrinsic for CD4⁺ T cells and were accompanied by a reduction of CD8⁺ T cells, antibodies, and a severe failure in viral control. We identified IL-27 as a gp130 cytokine that promoted antiviral CD4⁺ T cell accumulation in vivo and that rapidly induced IL-21 ex vivo. Furthermore, IL-27R was critical for control of persistent LCMV in vivo. These results reveal that gp130 cytokines (particularly IL-27) are key regulators of CD4⁺ T cell responses during an established chronic viral infection, empowering both humoral and cytotoxic immunity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
IL-6 and IL-27 play both distinct and redundant roles in regulating CD4 T-cell responses during chronic viral infection.Front Immunol. 2023 Jul 31;14:1221562. doi: 10.3389/fimmu.2023.1221562. eCollection 2023. Front Immunol. 2023. PMID: 37583704 Free PMC article.
-
Interleukin-27R Signaling Mediates Early Viral Containment and Impacts Innate and Adaptive Immunity after Chronic Lymphocytic Choriomeningitis Virus Infection.J Virol. 2018 May 29;92(12):e02196-17. doi: 10.1128/JVI.02196-17. Print 2018 Jun 15. J Virol. 2018. PMID: 29593047 Free PMC article.
-
Cell-Intrinsic gp130 Signaling on CD4+ T Cells Shapes Long-Lasting Antiviral Immunity.J Immunol. 2015 Aug 1;195(3):1071-81. doi: 10.4049/jimmunol.1402402. Epub 2015 Jun 17. J Immunol. 2015. PMID: 26085685 Free PMC article.
-
Chronic LCMV Infection Is Fortified with Versatile Tactics to Suppress Host T Cell Immunity and Establish Viral Persistence.Viruses. 2021 Sep 29;13(10):1951. doi: 10.3390/v13101951. Viruses. 2021. PMID: 34696381 Free PMC article. Review.
-
IL-27 expression regulation and its effects on adaptive immunity against viruses.Front Immunol. 2024 Jun 20;15:1395921. doi: 10.3389/fimmu.2024.1395921. eCollection 2024. Front Immunol. 2024. PMID: 38966644 Free PMC article. Review.
Cited by
-
IL-6 and IL-27 play both distinct and redundant roles in regulating CD4 T-cell responses during chronic viral infection.Front Immunol. 2023 Jul 31;14:1221562. doi: 10.3389/fimmu.2023.1221562. eCollection 2023. Front Immunol. 2023. PMID: 37583704 Free PMC article.
-
C1q restrains autoimmunity and viral infection by regulating CD8+ T cell metabolism.Science. 2018 May 4;360(6388):558-563. doi: 10.1126/science.aao4555. Science. 2018. PMID: 29724957 Free PMC article.
-
IL-27 promotes the expansion of self-renewing CD8+ T cells in persistent viral infection.J Exp Med. 2019 Aug 5;216(8):1791-1808. doi: 10.1084/jem.20190173. Epub 2019 Jun 4. J Exp Med. 2019. PMID: 31164392 Free PMC article.
-
IL-27 limits central nervous system viral clearance by promoting IL-10 and enhances demyelination.J Immunol. 2014 Jul 1;193(1):285-94. doi: 10.4049/jimmunol.1400058. Epub 2014 Jun 2. J Immunol. 2014. PMID: 24890725 Free PMC article.
-
Selection on Network Dynamics Drives Differential Rates of Protein Domain Evolution.PLoS Genet. 2016 Jul 5;12(7):e1006132. doi: 10.1371/journal.pgen.1006132. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27380265 Free PMC article.
References
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
-
- Benmira S, Bhattacharya V, Schmid ML. An effective HIV vaccine: A combination of humoral and cellular immunity? Curr HIV Res. 2010;8:441–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

