HLA-B27 misfolding and ankylosing spondylitis
- PMID: 23993278
- PMCID: PMC3857088
- DOI: 10.1016/j.molimm.2013.07.013
HLA-B27 misfolding and ankylosing spondylitis
Abstract
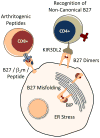
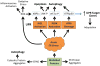
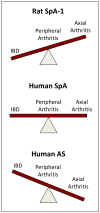
Understanding how HLA-B27 contributes to the pathogenesis of spondyloarthritis continues to be an important goal. Current efforts are aimed largely on three areas of investigation; peptide presentation to CD8T cells, abnormal forms of the HLA-B27 heavy chain and their recognition by leukocyte immunoglobulin-like receptors on immune effector cells, and HLA-B27 heavy chain misfolding and intrinsic biological effects on affected cells. In this chapter we review our current understanding of the causes and consequences of HLA-B27 misfolding, which can be defined biochemically as a propensity to oligomerize and form complexes in the endoplasmic reticulum (ER) with the chaperone BiP (HSPA5/GRP78). HLA-B27 misfolding is linked to an unusual combination of polymorphisms that identify this allele, and cause the heavy chain to fold and load peptides inefficiently. Misfolding can result in ER-associated degradation (ERAD) of heavy chains, which is mediated in part by the E3 ubiquitin ligase HRD1 (SYVN1), and the ubiquitin conjugating enzyme UBE2JL. Upregulation of HLA-B27 and accumulation of misfolded heavy chains can activate ER stress signaling pathways that orchestrate the unfolded protein response. In transgenic rats where HLA-B27 is overexpressed, UPR activation is prominent. However, it is specific for heavy chain misfolding, since overexpression of HLA-B7, an allele that does not misfold, fails to generate ER stress. UPR activation has been linked to cytokine dysregulation, promoting lL-23, IFNβ, and lL-1α production, and may activate the IL-23/IL-17 axis in these rats. IL-1α and IFNβ are pro- and anti-osteoclastogenic cytokines, respectively, that modulate osteoclast development in HLA-B27-expressing transgenic rat monocytes. Translational studies of patient derived cells expressing HLA-B27 at physiologic levels have provided evidence that ER stress and UPR activation can occur in peripheral blood, but this has not been reported to date in isolated macrophages. Inflamed gastrointestinal tissue reveals evidence for HLA-B27 misfolding, ERAD, and autophagy, without acute UPR activation. A more complete picture of conditions that impact HLA-B27 folding and misfolding, the full spectrum and time course of consequences of ER stress, and critical cell types involved is needed to understand the role of HLA-B27 misfolding in spondyloarthritis pathogenesis.
Keywords: Ankylosing spondylitis; Autophagy; Endoplasmic reticulum stress; MHC class I; Protein misfolding; Spondyloarthritis; Unfolded protein response.
Published by Elsevier Ltd.
Figures
Similar articles
-
HLA-B27 misfolding and spondyloarthropathies.Prion. 2009 Jan-Mar;3(1):15-26. doi: 10.4161/pri.3.1.8072. Epub 2009 Jan 3. Prion. 2009. PMID: 19363299 Free PMC article. Review.
-
HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response.J Immunol. 2005 Aug 15;175(4):2438-48. doi: 10.4049/jimmunol.175.4.2438. J Immunol. 2005. PMID: 16081815
-
HLA-B27 up-regulation causes accumulation of misfolded heavy chains and correlates with the magnitude of the unfolded protein response in transgenic rats: Implications for the pathogenesis of spondylarthritis-like disease.Arthritis Rheum. 2007 Jan;56(1):215-23. doi: 10.1002/art.22295. Arthritis Rheum. 2007. PMID: 17195225
-
HLA-B27 misfolding and spondyloarthropathies.Adv Exp Med Biol. 2009;649:217-34. doi: 10.1007/978-1-4419-0298-6_16. Adv Exp Med Biol. 2009. PMID: 19731632 Review.
-
HLA-B27 alters the response to tumor necrosis factor α and promotes osteoclastogenesis in bone marrow monocytes from HLA-B27-transgenic rats.Arthritis Rheum. 2013 Aug;65(8):2123-31. doi: 10.1002/art.38001. Arthritis Rheum. 2013. PMID: 23666508 Free PMC article.
Cited by
-
IL-23/IL-17 axis in spondyloarthritis-bench to bedside.Clin Rheumatol. 2016 Jun;35(6):1437-41. doi: 10.1007/s10067-016-3263-4. Epub 2016 Apr 13. Clin Rheumatol. 2016. PMID: 27075462 Review.
-
Ultrasonography of heel entheses in axial spondyloarthritis patients: frequency and assessment of associated factors.J Ultrasound. 2023 Mar;26(1):185-192. doi: 10.1007/s40477-022-00715-x. Epub 2022 Sep 6. J Ultrasound. 2023. PMID: 36068431 Free PMC article.
-
How Has Molecular Biology Enhanced Our Undertaking of axSpA and Its Management.Curr Rheumatol Rep. 2023 Jan;25(1):12-33. doi: 10.1007/s11926-022-01092-4. Epub 2022 Oct 29. Curr Rheumatol Rep. 2023. PMID: 36308677 Free PMC article. Review.
-
Expression analysis of HLA-E and NKG2A and NKG2C receptors points at a role for natural killer function in ankylosing spondylitis.RMD Open. 2018 Jul 13;4(2):e000597. doi: 10.1136/rmdopen-2017-000597. eCollection 2018. RMD Open. 2018. PMID: 30018803 Free PMC article.
-
Combining Understanding of Immunological Mechanisms and Genetic Variants Toward Development of Personalized Medicine for Psoriasis Patients.Front Genet. 2019 May 3;10:395. doi: 10.3389/fgene.2019.00395. eCollection 2019. Front Genet. 2019. PMID: 31130981 Free PMC article. Review.
References
-
- Allen RL, O’Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–5048. - PubMed
-
- Antoniou AN, Ford S, Taurog JD, Butcher GW, Powis SJ. Formation of HLA-B27 homodimers and their relationship to assembly kinetics. J Biol Chem. 2004;279:8895–8902. - PubMed
-
- Benjamin RJ, Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990;11:137–142. - PubMed
-
- Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003;33:748–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

