Human glioblastoma multiforme: p53 reactivation by a novel MDM2 inhibitor
- PMID: 23977270
- PMCID: PMC3747081
- DOI: 10.1371/journal.pone.0072281
Human glioblastoma multiforme: p53 reactivation by a novel MDM2 inhibitor
Abstract
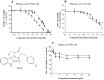
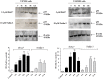
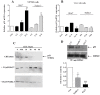
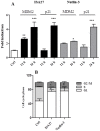
Cancer development and chemo-resistance are often due to impaired functioning of the p53 tumor suppressor through genetic mutation or sequestration by other proteins. In glioblastoma multiforme (GBM), p53 availability is frequently reduced because it binds to the Murine Double Minute-2 (MDM2) oncoprotein, which accumulates at high concentrations in tumor cells. The use of MDM2 inhibitors that interfere with the binding of p53 and MDM2 has become a valid approach to inhibit cell growth in a number of cancers; however little is known about the efficacy of these inhibitors in GBM. We report that a new small-molecule inhibitor of MDM2 with a spirooxoindolepyrrolidine core structure, named ISA27, effectively reactivated p53 function and inhibited human GBM cell growth in vitro by inducing cell cycle arrest and apoptosis. In immunoincompetent BALB/c nude mice bearing a human GBM xenograft, the administration of ISA27 in vivo activated p53, inhibited cell proliferation and induced apoptosis in tumor tissue. Significantly, ISA27 was non-toxic in an in vitro normal human cell model and an in vivo mouse model. ISA27 administration in combination with temozolomide (TMZ) produced a synergistic inhibitory effect on GBM cell viability in vitro, suggesting the possibility of lowering the dose of TMZ used in the treatment of GBM. In conclusion, our data show that ISA27 releases the powerful antitumor capacities of p53 in GBM cells. The use of this MDM2 inhibitor could become a novel therapy for the treatment of GBM patients.
Conflict of interest statement
Figures

Similar articles
-
CXCR4 antagonism sensitizes cancer cells to novel indole-based MDM2/4 inhibitors in glioblastoma multiforme.Eur J Pharmacol. 2021 Apr 15;897:173936. doi: 10.1016/j.ejphar.2021.173936. Epub 2021 Feb 10. Eur J Pharmacol. 2021. PMID: 33581134
-
Targeted Brain Tumor Therapy by Inhibiting the MDM2 Oncogene: In Vitro and In Vivo Antitumor Activity and Mechanism of Action.Cells. 2020 Jul 1;9(7):1592. doi: 10.3390/cells9071592. Cells. 2020. PMID: 32630235 Free PMC article.
-
A stapled peptide antagonist of MDM2 carried by polymeric micelles sensitizes glioblastoma to temozolomide treatment through p53 activation.J Control Release. 2015 Nov 28;218:29-35. doi: 10.1016/j.jconrel.2015.09.061. Epub 2015 Sep 30. J Control Release. 2015. PMID: 26428461 Free PMC article.
-
Spiro-oxindoles as a Promising Class of Small Molecule Inhibitors of p53-MDM2 Interaction Useful in Targeted Cancer Therapy.Top Curr Chem (Cham). 2017 Feb;375(1):3. doi: 10.1007/s41061-016-0089-0. Epub 2016 Dec 9. Top Curr Chem (Cham). 2017. PMID: 27943171 Review.
-
MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer.J Hematol Oncol. 2017 Jul 3;10(1):133. doi: 10.1186/s13045-017-0500-5. J Hematol Oncol. 2017. PMID: 28673313 Free PMC article. Review.
Cited by
-
Antioxidant and Antisenescence Effects of Bergamot Juice.Oxid Med Cell Longev. 2018 Jul 12;2018:9395804. doi: 10.1155/2018/9395804. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30116497 Free PMC article.
-
Oxidative Stress Activated by Sorafenib Alters the Temozolomide Sensitivity of Human Glioma Cells Through Autophagy and JAK2/STAT3-AIF Axis.Front Cell Dev Biol. 2021 Jun 14;9:660005. doi: 10.3389/fcell.2021.660005. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34277607 Free PMC article.
-
The MDM2 small-molecule inhibitor RG7388 leads to potent tumor inhibition in p53 wild-type neuroblastoma.Cell Death Discov. 2015;1:15026-. doi: 10.1038/cddiscovery.2015.26. Epub 2015 Aug 24. Cell Death Discov. 2015. PMID: 26998348 Free PMC article.
-
Evaluation of JC and Cytomegalo Viruses in Glioblastoma Tissue.Asian Pac J Cancer Prev. 2016 Nov 1;17(11):4907-4911. doi: 10.22034/APJCP.2016.17.11.4907. Asian Pac J Cancer Prev. 2016. PMID: 28032494 Free PMC article.
-
New small molecules, ISA27 and SM13, inhibit tumour growth inducing mitochondrial effects of p53.Br J Cancer. 2015 Jan 6;112(1):77-85. doi: 10.1038/bjc.2014.577. Epub 2014 Nov 25. Br J Cancer. 2015. PMID: 25422906 Free PMC article.
References
-
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, et al. (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21: 2683–2710. - PubMed
-
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, et al. (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64: 6892–6899. - PubMed
-
- Conrad CA, Milosavljevic VP, Yung WK (1995) Advances in chemotherapy for brain tumors. Neurol Clin 13: 795–812. - PubMed
-
- Levin VA, Leibel SA, Gutin PH (2001) Neoplasms of the central nervous system. In: De Vita Jr VT, Hellman S, Rosenberg SA, editors. Cancer principles of oncology. Philadelphia: Lippincott-Raven. 2100–2161.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

