Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways
- PMID: 23968377
- PMCID: PMC3978638
- DOI: 10.1186/ar4274
Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways
Abstract
Introduction: Intervertebral disc (IVD) degeneration is characterized by extracellular matrix breakdown and is considered to be a primary cause of discogenic back pain. Although increases in pro-inflammatory cytokine levels within degenerating discs are associated with discogenic back pain, the mechanisms leading to their overproduction have not yet been elucidated. As fragmentation of matrix components occurs during IVD degeneration, we assessed the potential involvement of hyaluronic acid fragments (fHAs) in the induction of inflammatory and catabolic mediators.
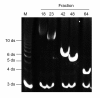
Methods: Human IVD cells isolated from patient biopsies were stimulated with fHAs (6 to 12 disaccharides) and their effect on cytokine and matrix degrading enzyme production was assessed using quantitative real-time polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA). The involvement of specific cell surface receptors and signal transduction pathways in mediating the effects of fHAs was tested using small interfering RNA (siRNA) approaches and kinase inhibition assays.
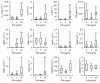
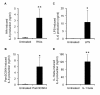
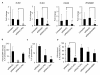
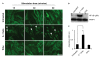
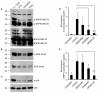
Results: Treatment of IVD cells with fHAs significantly increased mRNA expression levels of interleukin (IL)-1β, IL-6, IL-8, cyclooxygenase (COX)-2, matrix metalloproteinase (MMP)-1 and -13. The stimulatory effects of fHAs on IL-6 protein production were significantly impaired when added to IVD cells in combination with either Toll-like receptor (TLR)-2 siRNA or a TLR2 neutralizing antibody. Furthermore, the ability of fHAs to enhance IL-6 and MMP-3 protein production was found to be dependent on the mitogen-activated protein (MAP) kinase signaling pathway.
Conclusions: These findings suggest that fHAs may have the potential to mediate IVD degeneration and discogenic back pain through activation of the TLR2 signaling pathway in resident IVD cells.
Figures
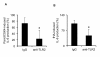
Similar articles
-
IL-1β/HMGB1 signalling promotes the inflammatory cytokines release via TLR signalling in human intervertebral disc cells.Biosci Rep. 2016 Sep 16;36(5):e00379. doi: 10.1042/BSR20160118. Print 2016 Oct. Biosci Rep. 2016. PMID: 27512095 Free PMC article.
-
Toll-Like Receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration.Spine (Phila Pa 1976). 2013 Jul 15;38(16):1343-51. doi: 10.1097/BRS.0b013e31826b71f4. Spine (Phila Pa 1976). 2013. PMID: 22850250
-
Sesamin inhibits lipopolysaccharide-induced inflammation and extracellular matrix catabolism in rat intervertebral disc.Connect Tissue Res. 2016 Sep;57(5):347-59. doi: 10.1080/03008207.2016.1182998. Epub 2016 Apr 29. Connect Tissue Res. 2016. PMID: 27128308
-
For whom the disc tolls: intervertebral disc degeneration, back pain and toll-like receptors.Eur Cell Mater. 2021 Mar 19;41:355-369. doi: 10.22203/eCM.v041a23. Eur Cell Mater. 2021. PMID: 33738788 Review.
-
MAPK /ERK signaling pathway: A potential target for the treatment of intervertebral disc degeneration.Biomed Pharmacother. 2021 Nov;143:112170. doi: 10.1016/j.biopha.2021.112170. Epub 2021 Sep 15. Biomed Pharmacother. 2021. PMID: 34536759 Review.
Cited by
-
Mechanobiology of MicroRNAs in Intervertebral Disk Degeneration.J Spine Res Surg. 2023;5(1):1-9. doi: 10.26502/fjsrs0051. Epub 2023 Jan 17. J Spine Res Surg. 2023. PMID: 36777190 Free PMC article.
-
Extracellular matrix in intervertebral disc: basic and translational implications.Cell Tissue Res. 2022 Oct;390(1):1-22. doi: 10.1007/s00441-022-03662-5. Epub 2022 Jul 6. Cell Tissue Res. 2022. PMID: 35792910 Review.
-
Hyaluronan in the experimental injury of the cartilage: biochemical action and protective effects.Inflamm Res. 2018 Jan;67(1):5-20. doi: 10.1007/s00011-017-1084-9. Epub 2017 Aug 12. Inflamm Res. 2018. PMID: 28803264 Review.
-
Circular RNA and intervertebral disc degeneration: unravelling mechanisms and implications.Front Mol Biosci. 2023 Dec 19;10:1302017. doi: 10.3389/fmolb.2023.1302017. eCollection 2023. Front Mol Biosci. 2023. PMID: 38192334 Free PMC article. Review.
-
The circular RNA circ-GRB10 participates in the molecular circuitry inhibiting human intervertebral disc degeneration.Cell Death Dis. 2020 Aug 13;11(8):612. doi: 10.1038/s41419-020-02882-3. Cell Death Dis. 2020. PMID: 32792505 Free PMC article.
References
-
- De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ. The role of interleukin-6 in nociception and pain. Anesth Analg. 2003;15:1096–1103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

