Neutralizing IgG at the portal of infection mediates protection against vaginal simian/human immunodeficiency virus challenge
- PMID: 23966410
- PMCID: PMC3807318
- DOI: 10.1128/JVI.01361-13
Neutralizing IgG at the portal of infection mediates protection against vaginal simian/human immunodeficiency virus challenge
Abstract
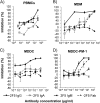
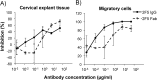
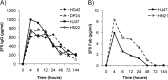
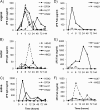
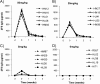
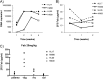
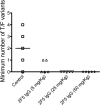
Neutralizing antibodies may have critical importance in immunity against human immunodeficiency virus type 1 (HIV-1) infection. However, the amount of protective antibody needed at mucosal surfaces has not been fully established. Here, we evaluated systemic and mucosal pharmacokinetics (PK) and pharmacodynamics (PD) of 2F5 IgG and 2F5 Fab fragments with respect to protection against vaginal challenge with simian-human immunodeficiency virus-BaL in macaques. Antibody assessment demonstrated that 2F5 IgG was more potent than polymeric forms (IgM and IgA) across a range of cellular and tissue models. Vaginal challenge studies demonstrated a dose-dependent protection for 2F5 IgG and no protection with 2F5 Fab despite higher vaginal Fab levels at the time of challenge. Animals receiving 50 or 25 mg/kg of body weight 2F5 IgG were completely protected, while 3/5 animals receiving 5 mg/kg were protected. In the control animals, infection was established by a minimum of 1 to 4 transmitted/founder (T/F) variants, similar to natural human infection by this mucosal route; in the two infected animals that had received 5 mg 2F5 IgG, infection was established by a single T/F variant. Serum levels of 2F5 IgG were more predictive of sterilizing protection than measured vaginal levels. Fc-mediated antiviral activity did not appear to influence infection of primary target cells in cervical explants. However, PK studies highlighted the importance of the Fc portion in tissue biodistribution. Data presented in this study may be important in modeling serum levels of neutralizing antibodies that need to be achieved by either vaccination or passive infusion to prevent mucosal acquisition of HIV-1 infection in humans.
Figures



Similar articles
-
Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge.Vaccine. 2015 Apr 21;33(17):2086-95. doi: 10.1016/j.vaccine.2015.02.020. Epub 2015 Mar 11. Vaccine. 2015. PMID: 25769884 Free PMC article.
-
Cellular immunity elicited by human immunodeficiency virus type 1/ simian immunodeficiency virus DNA vaccination does not augment the sterile protection afforded by passive infusion of neutralizing antibodies.J Virol. 2003 Oct;77(19):10348-56. doi: 10.1128/jvi.77.19.10348-10356.2003. J Virol. 2003. PMID: 12970419 Free PMC article.
-
Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies.Nat Med. 2000 Feb;6(2):207-10. doi: 10.1038/72318. Nat Med. 2000. PMID: 10655111
-
Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1.Vaccine. 2002 May 6;20(15):1922-5. doi: 10.1016/s0264-410x(02)00068-3. Vaccine. 2002. PMID: 11983246 Review.
-
Fc receptor-mediated antiviral antibodies.Curr Opin HIV AIDS. 2009 Sep;4(5):388-93. doi: 10.1097/COH.0b013e32832f0a89. Curr Opin HIV AIDS. 2009. PMID: 20048702 Free PMC article. Review.
Cited by
-
Anatomic Distribution of Intravenously Injected IgG Takes Approximately 1 Week to Achieve Stratum Corneum Saturation in Vaginal Tissues.J Immunol. 2021 Jul 15;207(2):505-511. doi: 10.4049/jimmunol.2100253. Epub 2021 Jun 23. J Immunol. 2021. PMID: 34162723 Free PMC article.
-
Enhanced neonatal Fc receptor function improves protection against primate SHIV infection.Nature. 2014 Oct 30;514(7524):642-5. doi: 10.1038/nature13612. Epub 2014 Aug 13. Nature. 2014. PMID: 25119033 Free PMC article.
-
MABGEL 1: first phase 1 trial of the anti-HIV-1 monoclonal antibodies 2F5, 4E10 and 2G12 as a vaginal microbicide.PLoS One. 2014 Dec 29;9(12):e116153. doi: 10.1371/journal.pone.0116153. eCollection 2014. PLoS One. 2014. PMID: 25546420 Free PMC article. Clinical Trial.
-
HIV Broadly Neutralizing Antibodies Expressed as IgG3 Preserve Neutralization Potency and Show Improved Fc Effector Function.Front Immunol. 2021 Sep 10;12:733958. doi: 10.3389/fimmu.2021.733958. eCollection 2021. Front Immunol. 2021. PMID: 34566999 Free PMC article.
-
Mucosal application of the broadly neutralizing antibody 10-1074 protects macaques from cell-associated SHIV vaginal exposure.Nat Commun. 2023 Oct 6;14(1):6224. doi: 10.1038/s41467-023-41966-4. Nat Commun. 2023. PMID: 37803011 Free PMC article.
References
-
- Haase AT. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783–792 - PubMed
-
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 - PubMed
-
- Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433.10.1371/journal.ppat.1000433 - DOI - PMC - PubMed
-
- Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009–4018 - PMC - PubMed
-
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

