Immunotoxins: the role of the toxin
- PMID: 23965432
- PMCID: PMC3760048
- DOI: 10.3390/toxins5081486
Immunotoxins: the role of the toxin
Abstract
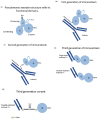
Immunotoxins are antibody-toxin bifunctional molecules that rely on intracellular toxin action to kill target cells. Target specificity is determined via the binding attributes of the chosen antibody. Mostly, but not exclusively, immunotoxins are purpose-built to kill cancer cells as part of novel treatment approaches. Other applications for immunotoxins include immune regulation and the treatment of viral or parasitic diseases. Here we discuss the utility of protein toxins, of both bacterial and plant origin, joined to antibodies for targeting cancer cells. Finally, while clinical goals are focused on the development of novel cancer treatments, much has been learned about toxin action and intracellular pathways. Thus toxins are considered both medicines for treating human disease and probes of cellular function.
Figures

Similar articles
-
Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy.Cancer Sci. 2009 Aug;100(8):1359-65. doi: 10.1111/j.1349-7006.2009.01192.x. Epub 2009 May 19. Cancer Sci. 2009. PMID: 19459847 Free PMC article. Review.
-
Recombinant immunotoxins containing truncated bacterial toxins for the treatment of hematologic malignancies.BioDrugs. 2009;23(1):1-13. doi: 10.2165/00063030-200923010-00001. BioDrugs. 2009. PMID: 19344187 Free PMC article. Review.
-
Anti-cancer Immunotoxins, Challenges, and Approaches.Curr Pharm Des. 2021;27(7):932-941. doi: 10.2174/1381612826666201006155346. Curr Pharm Des. 2021. PMID: 33023437
-
Immunotoxins.Biotherapy. 1991;3(1):65-76. doi: 10.1007/BF02175100. Biotherapy. 1991. PMID: 2009215 Review.
-
Patents on immunotoxins and chimeric toxins for the treatment of cancer.Recent Pat Drug Deliv Formul. 2007;1(2):105-15. doi: 10.2174/187221107780831932. Recent Pat Drug Deliv Formul. 2007. PMID: 19075878 Review.
Cited by
-
Bortezomib reduces pre-existing antibodies to recombinant immunotoxins in mice.J Immunol. 2015 Feb 15;194(4):1695-701. doi: 10.4049/jimmunol.1402324. Epub 2015 Jan 5. J Immunol. 2015. PMID: 25560410 Free PMC article.
-
Rationally designed chemokine-based toxin targeting the viral G protein-coupled receptor US28 potently inhibits cytomegalovirus infection in vivo.Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):8427-32. doi: 10.1073/pnas.1509392112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080445 Free PMC article.
-
A Novel EGFR Targeted Immunotoxin Based on Cetuximab and Type 1 RIP Quinoin Overcomes the Cetuximab Resistance in Colorectal Cancer Cells.Toxins (Basel). 2023 Jan 9;15(1):57. doi: 10.3390/toxins15010057. Toxins (Basel). 2023. PMID: 36668877 Free PMC article.
-
A chemical conjugate between HER2-targeting antibody fragment and Pseudomonas exotoxin A fragment demonstrates cytotoxic effects on HER2-expressing breast cancer cells.BMB Rep. 2019 Aug;52(8):496-501. doi: 10.5483/BMBRep.2019.52.8.250. BMB Rep. 2019. PMID: 30670149 Free PMC article.
-
Recombinant Immunotoxin Therapy of Solid Tumors: Challenges and Strategies.J Basic Clin Med. 2013;2(2):1-6. J Basic Clin Med. 2013. PMID: 25309827 Free PMC article.
References
-
- Vitetta E.S., Krolick K.A., Miyama-Inaba M., Cushley W., Uhr J.W. Immunotoxins: A new approach to cancer therapy. Science. 1983;219:644–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

