Supplementation with fish oil and genistein, individually or in combination, protects bone against the adverse effects of methotrexate chemotherapy in rats
- PMID: 23951199
- PMCID: PMC3741109
- DOI: 10.1371/journal.pone.0071592
Supplementation with fish oil and genistein, individually or in combination, protects bone against the adverse effects of methotrexate chemotherapy in rats
Abstract
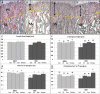
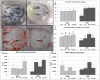
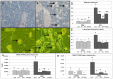
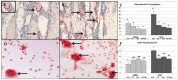
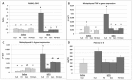
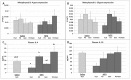
Cancer chemotherapy has been shown to induce long-term skeletal side effects such as osteoporosis and fractures; however, there are no preventative treatments. This study investigated the damaging effects of anti-metabolite methotrexate (MTX) subcutaneous injections (0.75 mg/kg BW) for five days and the potential protective benefits of daily oral gavage of fish oil at 0.5 mL/100 g BW (containing 375 mg of n-3 PUFA/100 g BW), genistein (2 mg/100 g BW), or their combination in young adult rats. MTX treatment alone significantly reduced primary spongiosa height and secondary spongiosa trabecular bone volume. Bone marrow stromal cells from the treated rats showed a significant reduction in osteogenic differentiation but an increase in adipogenesis ex vivo. Consistently, stromal cells had significantly higher mRNA levels of adipogenesis-related proliferator activator activated receptor-γ (PPAR-γ) and fatty acid binding protein (FABP4). MTX significantly increased the numbers of bone-resorbing osteoclasts and marrow osteoclast precursor cell pool while significantly enhancing the mRNA expression of receptor activator for nuclear factor kappa B ligand (RANKL), the RANKL/osteoprotegerin (OPG) ratio, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the bone. Supplementary treatment with fish oil and/or genistein significantly preserved trabecular bone volume and osteogenesis but suppressed MTX-induced adipogenesis and increases in osteoclast numbers and pro-osteoclastogenic cytokine expression. Thus, Fish oil and/or genistein supplementation during MTX treatment enabled not only preservation of osteogenic differentiation, osteoblast number and bone volume, but also prevention of MTX treatment-induced increases in bone marrow adiposity, osteoclastogenic cytokine expression and osteoclast formation, and thus bone loss.
Conflict of interest statement
Figures
Similar articles
-
Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats.Int J Mol Sci. 2015 Aug 6;16(8):18293-311. doi: 10.3390/ijms160818293. Int J Mol Sci. 2015. PMID: 26258775 Free PMC article.
-
Fish oil in comparison to folinic acid for protection against adverse effects of methotrexate chemotherapy on bone.J Orthop Res. 2014 Apr;32(4):587-96. doi: 10.1002/jor.22565. Epub 2013 Dec 17. J Orthop Res. 2014. PMID: 24346859
-
Effects of Resveratrol Supplementation on Methotrexate Chemotherapy-Induced Bone Loss.Nutrients. 2017 Mar 9;9(3):255. doi: 10.3390/nu9030255. Nutrients. 2017. PMID: 28282956 Free PMC article.
-
Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling.J Cell Physiol. 2013 Mar;228(3):513-21. doi: 10.1002/jcp.24158. J Cell Physiol. 2013. PMID: 22777826 Review.
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
Cited by
-
Osteoblast derived-neurotrophin‑3 induces cartilage removal proteases and osteoclast-mediated function at injured growth plate in rats.Bone. 2018 Nov;116:232-247. doi: 10.1016/j.bone.2018.08.010. Epub 2018 Aug 17. Bone. 2018. PMID: 30125729 Free PMC article.
-
Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Protects Against Adriamycin and Cyclophosphamide Chemotherapy-Induced Bone Marrow Damage in Female Rats.Int J Mol Sci. 2018 Feb 6;19(2):484. doi: 10.3390/ijms19020484. Int J Mol Sci. 2018. PMID: 29415482 Free PMC article.
-
ω-3 Long Chain Polyunsaturated Fatty Acids as Sensitizing Agents and Multidrug Resistance Revertants in Cancer Therapy.Int J Mol Sci. 2017 Dec 20;18(12):2770. doi: 10.3390/ijms18122770. Int J Mol Sci. 2017. PMID: 29261109 Free PMC article. Review.
-
Potential Effects of Phytoestrogen Genistein in Modulating Acute Methotrexate Chemotherapy-Induced Osteoclastogenesis and Bone Damage in Rats.Int J Mol Sci. 2015 Aug 6;16(8):18293-311. doi: 10.3390/ijms160818293. Int J Mol Sci. 2015. PMID: 26258775 Free PMC article.
-
Restraining the Proliferation of Acute Lymphoblastic Leukemia Cells by Genistein through Up-regulation of B-cell Translocation Gene-3 at Transcription Level.Galen Med J. 2019 Mar 26;8:e1229. doi: 10.31661/gmj.v8i0.1229. eCollection 2019. Galen Med J. 2019. PMID: 34466474 Free PMC article.
References
-
- Fan C, Cool JC, Scherer MA, Foster BK, Shandala T, et al. (2009) Damaging effects of chronic low-dose methotrexate usage on primary bone formation in young rats and potential protective effects of folinic acid supplementary treatment. Bone 44: 61–70. - PubMed
-
- Xian CJ, Cool JC, Pyragius T, Foster BK (2006) Damage and recovery of the bone growth mechanism in young rats following 5-fluorouracil acute chemotherapy. J Cell Biochem 99: 1688–1704. - PubMed
-
- Xian CJ, Cool JC, Scherer MA, Macsai CE, Fan C, et al. (2007) Cellular mechanisms for methotrexate chemotherapy-induced bone growth defects. Bone 41: 842–850. - PubMed
-
- Xian CJ, Cool JC, van Gangelen J, Foster BK, Howarth GS (2007) Effects of etoposide and cyclophosphamide acute chemotherapy on growth plate and metaphyseal bone in rats. Cancer Biol Ther 6: 170–177. - PubMed
-
- Atkinson SA, Fraher L, Gundberg CM, Andrew M, Pai M, et al. (1989) Mineral homeostasis and bone mass in children treated for acute lymphoblastic leukemia. J Pediatr 114: 793–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

