Immunohistochemical, ultrastructural and functional analysis of axonal regeneration through peripheral nerve grafts containing Schwann cells expressing BDNF, CNTF or NT3
- PMID: 23950907
- PMCID: PMC3739754
- DOI: 10.1371/journal.pone.0069987
Immunohistochemical, ultrastructural and functional analysis of axonal regeneration through peripheral nerve grafts containing Schwann cells expressing BDNF, CNTF or NT3
Abstract
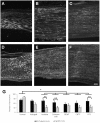
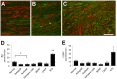
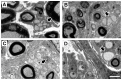
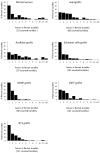
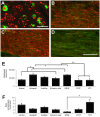
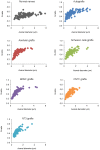
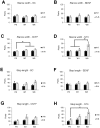
We used morphological, immunohistochemical and functional assessments to determine the impact of genetically-modified peripheral nerve (PN) grafts on axonal regeneration after injury. Grafts were assembled from acellular nerve sheaths repopulated ex vivo with Schwann cells (SCs) modified to express brain-derived neurotrophic factor (BDNF), a secretable form of ciliary neurotrophic factor (CNTF), or neurotrophin-3 (NT3). Grafts were used to repair unilateral 1 cm defects in rat peroneal nerves and 10 weeks later outcomes were compared to normal nerves and various controls: autografts, acellular grafts and grafts with unmodified SCs. The number of regenerated βIII-Tubulin positive axons was similar in all grafts with the exception of CNTF, which contained the fewest immunostained axons. There were significantly lower fiber counts in acellular, untransduced SC and NT3 groups using a PanNF antibody, suggesting a paucity of large caliber axons. In addition, NT3 grafts contained the greatest number of sensory fibres, identified with either IB4 or CGRP markers. Examination of semi- and ultra-thin sections revealed heterogeneous graft morphologies, particularly in BDNF and NT3 grafts in which the fascicular organization was pronounced. Unmyelinated axons were loosely organized in numerous Remak bundles in NT3 grafts, while the BDNF graft group displayed the lowest ratio of umyelinated to myelinated axons. Gait analysis revealed that stance width was increased in rats with CNTF and NT3 grafts, and step length involving the injured left hindlimb was significantly greater in NT3 grafted rats, suggesting enhanced sensory sensitivity in these animals. In summary, the selective expression of BDNF, CNTF or NT3 by genetically modified SCs had differential effects on PN graft morphology, the number and type of regenerating axons, myelination, and locomotor function.
Conflict of interest statement
Figures

Similar articles
-
Regeneration of adult rat sensory and motor neuron axons through chimeric peroneal nerve grafts containing donor Schwann cells engineered to express different neurotrophic factors.Exp Neurol. 2020 Aug;330:113355. doi: 10.1016/j.expneurol.2020.113355. Epub 2020 May 15. Exp Neurol. 2020. PMID: 32422148
-
The importance of transgene and cell type on the regeneration of adult retinal ganglion cell axons within reconstituted bridging grafts.Exp Neurol. 2007 Oct;207(2):314-28. doi: 10.1016/j.expneurol.2007.07.001. Epub 2007 Aug 8. Exp Neurol. 2007. PMID: 17689533
-
Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration.Mol Ther. 2005 Jun;11(6):906-15. doi: 10.1016/j.ymthe.2005.01.016. Mol Ther. 2005. PMID: 15922961
-
Schwann Cell Role in Selectivity of Nerve Regeneration.Cells. 2020 Sep 20;9(9):2131. doi: 10.3390/cells9092131. Cells. 2020. PMID: 32962230 Free PMC article. Review.
-
Gene therapy and transplantation in the retinofugal pathway.Prog Brain Res. 2009;175:151-61. doi: 10.1016/S0079-6123(09)17510-6. Prog Brain Res. 2009. PMID: 19660654 Review.
Cited by
-
Novel Glial Cell Functions: Extensive Potency, Stem Cell-Like Properties, and Participation in Regeneration and Transdifferentiation.Front Cell Dev Biol. 2020 Aug 18;8:809. doi: 10.3389/fcell.2020.00809. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015034 Free PMC article. Review.
-
Gene delivery to rat and human Schwann cells and nerve segments: a comparison of AAV 1-9 and lentiviral vectors.Gene Ther. 2015 Oct;22(10):767-80. doi: 10.1038/gt.2015.47. Epub 2015 May 4. Gene Ther. 2015. PMID: 25938190
-
Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system.Mol Neurobiol. 2014 Dec;50(3):945-70. doi: 10.1007/s12035-014-8706-9. Epub 2014 Apr 22. Mol Neurobiol. 2014. PMID: 24752592 Review.
-
Differential effects of 670 and 830 nm red near infrared irradiation therapy: a comparative study of optic nerve injury, retinal degeneration, traumatic brain and spinal cord injury.PLoS One. 2014 Aug 8;9(8):e104565. doi: 10.1371/journal.pone.0104565. eCollection 2014. PLoS One. 2014. PMID: 25105800 Free PMC article.
-
Evaluation of two methods to isolate Schwann cells from murine sciatic nerve.J Neurosci Methods. 2020 Feb 1;331:108483. doi: 10.1016/j.jneumeth.2019.108483. Epub 2019 Nov 19. J Neurosci Methods. 2020. PMID: 31756398 Free PMC article.
References
-
- Lundborg G (2000) A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg 35A: 391–414. - PubMed
-
- Dahlin LB, Brandt J (2004) Basic science of peripheral nerve repair: wallerian degeneratioMJG LTn/growth cones. Oper Techn Orthopaed 14: 138–145.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

