A distinct role of Riplet-mediated K63-Linked polyubiquitination of the RIG-I repressor domain in human antiviral innate immune responses
- PMID: 23950712
- PMCID: PMC3738492
- DOI: 10.1371/journal.ppat.1003533
A distinct role of Riplet-mediated K63-Linked polyubiquitination of the RIG-I repressor domain in human antiviral innate immune responses
Abstract
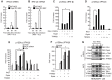
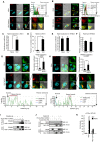
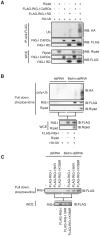
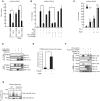
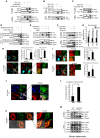
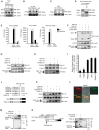
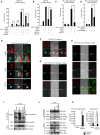
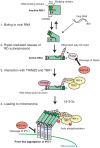
The innate immune system is essential for controlling viral infections, but several viruses have evolved strategies to escape innate immunity. RIG-I is a cytoplasmic viral RNA sensor that triggers the signal to induce type I interferon production in response to viral infection. RIG-I activation is regulated by the K63-linked polyubiquitin chain mediated by Riplet and TRIM25 ubiquitin ligases. TRIM25 is required for RIG-I oligomerization and interaction with the IPS-1 adaptor molecule. A knockout study revealed that Riplet was essential for RIG-I activation. However the molecular mechanism underlying RIG-I activation by Riplet remains unclear, and the functional differences between Riplet and TRIM25 are also unknown. A genetic study and a pull-down assay indicated that Riplet was dispensable for RIG-I RNA binding activity but required for TRIM25 to activate RIG-I. Mutational analysis demonstrated that Lys-788 within the RIG-I repressor domain was critical for Riplet-mediated K63-linked polyubiquitination and that Riplet was required for the release of RIG-I autorepression of its N-terminal CARDs, which leads to the association of RIG-I with TRIM25 ubiquitin ligase and TBK1 protein kinase. Our data indicate that Riplet is a prerequisite for TRIM25 to activate RIG-I signaling. We investigated the biological importance of this mechanism in human cells and found that hepatitis C virus (HCV) abrogated this mechanism. Interestingly, HCV NS3-4A proteases targeted the Riplet protein and abrogated endogenous RIG-I polyubiquitination and association with TRIM25 and TBK1, emphasizing the biological importance of this mechanism in human antiviral innate immunity. In conclusion, our results establish that Riplet-mediated K63-linked polyubiquitination released RIG-I RD autorepression, which allowed the access of positive factors to the RIG-I protein.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses.Front Immunol. 2020 Jun 24;11:1296. doi: 10.3389/fimmu.2020.01296. eCollection 2020. Front Immunol. 2020. PMID: 32670286 Free PMC article. Review.
-
Species-specific inhibition of RIG-I ubiquitination and IFN induction by the influenza A virus NS1 protein.PLoS Pathog. 2012;8(11):e1003059. doi: 10.1371/journal.ppat.1003059. Epub 2012 Nov 29. PLoS Pathog. 2012. PMID: 23209422 Free PMC article.
-
Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection.J Biol Chem. 2009 Jan 9;284(2):807-17. doi: 10.1074/jbc.M804259200. Epub 2008 Nov 18. J Biol Chem. 2009. PMID: 19017631
-
Human Respiratory Syncytial Virus NS 1 Targets TRIM25 to Suppress RIG-I Ubiquitination and Subsequent RIG-I-Mediated Antiviral Signaling.Viruses. 2018 Dec 14;10(12):716. doi: 10.3390/v10120716. Viruses. 2018. PMID: 30558248 Free PMC article.
-
Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I.J Biochem. 2012 Jan;151(1):5-11. doi: 10.1093/jb/mvr111. Epub 2011 Sep 2. J Biochem. 2012. PMID: 21890623 Review.
Cited by
-
Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses.Front Immunol. 2020 Jun 24;11:1296. doi: 10.3389/fimmu.2020.01296. eCollection 2020. Front Immunol. 2020. PMID: 32670286 Free PMC article. Review.
-
HPV Oncoproteins and the Ubiquitin Proteasome System: A Signature of Malignancy?Pathogens. 2020 Feb 18;9(2):133. doi: 10.3390/pathogens9020133. Pathogens. 2020. PMID: 32085533 Free PMC article. Review.
-
Evolution and expression of the duck TRIM gene repertoire.Front Immunol. 2023 Aug 9;14:1220081. doi: 10.3389/fimmu.2023.1220081. eCollection 2023. Front Immunol. 2023. PMID: 37622121 Free PMC article.
-
Viral Evasion Strategies in Type I IFN Signaling - A Summary of Recent Developments.Front Immunol. 2016 Nov 11;7:498. doi: 10.3389/fimmu.2016.00498. eCollection 2016. Front Immunol. 2016. PMID: 27891131 Free PMC article. Review.
-
Structural analysis of RIG-I-like receptors reveals ancient rules of engagement between diverse RNA helicases and TRIM ubiquitin ligases.Mol Cell. 2021 Feb 4;81(3):599-613.e8. doi: 10.1016/j.molcel.2020.11.047. Epub 2020 Dec 28. Mol Cell. 2021. PMID: 33373584 Free PMC article.
References
-
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, et al. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105. - PubMed
-
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, et al. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5: 730–737. - PubMed
-
- Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, et al. (2011) Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147: 423–435. - PubMed
-
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, et al. (2005) VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell 19: 727–740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

