Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix
- PMID: 23946487
- PMCID: PMC3790002
- DOI: 10.1074/jbc.M113.486753
Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix
Abstract
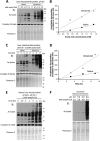
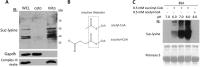
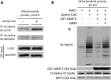
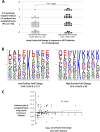
Alterations in mitochondrial protein acetylation are implicated in the pathophysiology of diabetes, the metabolic syndrome, mitochondrial disorders, and cancer. However, a viable mechanism responsible for the widespread acetylation in mitochondria remains unknown. Here, we demonstrate that the physiologic pH and acyl-CoA concentrations of the mitochondrial matrix are sufficient to cause dose- and time-dependent, but enzyme-independent acetylation and succinylation of mitochondrial and nonmitochondrial proteins in vitro. These data suggest that protein acylation in mitochondria may be a chemical event facilitated by the alkaline pH and high concentrations of reactive acyl-CoAs present in the mitochondrial matrix. Although these results do not exclude the possibility of enzyme-mediated protein acylation in mitochondria, they demonstrate that such a mechanism may not be required in its unique chemical environment. These findings may have implications for the evolutionary roles that the mitochondria-localized SIRT3 deacetylase and SIRT5 desuccinylase have in the maintenance of metabolic health.
Keywords: Acetyl Coenzyme A; Acetylation; Metabolic Diseases; Metabolic Regulation; Mitochondrial Metabolism; Nonenzymatic; Sirtuins; Succinyl Coenzyme A; Succinylation; pH Regulation.
Figures



Similar articles
-
The ɛ-Amino Group of Protein Lysine Residues Is Highly Susceptible to Nonenzymatic Acylation by Several Physiological Acyl-CoA Thioesters.Chembiochem. 2015 Nov 2;16(16):2337-47. doi: 10.1002/cbic.201500364. Epub 2015 Sep 18. Chembiochem. 2015. PMID: 26382620
-
Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease.J Biol Chem. 2012 Dec 14;287(51):42436-43. doi: 10.1074/jbc.R112.404863. Epub 2012 Oct 18. J Biol Chem. 2012. PMID: 23086951 Free PMC article. Review.
-
The Mitochondrial Acylome Emerges: Proteomics, Regulation by Sirtuins, and Metabolic and Disease Implications.Cell Metab. 2018 Mar 6;27(3):497-512. doi: 10.1016/j.cmet.2018.01.016. Cell Metab. 2018. PMID: 29514063 Free PMC article. Review.
-
The Role of Mitochondrial Non-Enzymatic Protein Acylation in Ageing.PLoS One. 2016 Dec 29;11(12):e0168752. doi: 10.1371/journal.pone.0168752. eCollection 2016. PLoS One. 2016. PMID: 28033361 Free PMC article.
-
Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5.J Mol Biol. 2008 Oct 10;382(3):790-801. doi: 10.1016/j.jmb.2008.07.048. Epub 2008 Jul 25. J Mol Biol. 2008. PMID: 18680753
Cited by
-
Acylspermidines are conserved mitochondrial sirtuin-dependent metabolites.Nat Chem Biol. 2024 Jul;20(7):812-822. doi: 10.1038/s41589-023-01511-2. Epub 2024 Jan 2. Nat Chem Biol. 2024. PMID: 38167917
-
MitomiRs: their roles in mitochondria and importance in cancer cell metabolism.Radiol Oncol. 2021 Nov 19;55(4):379-392. doi: 10.2478/raon-2021-0042. Radiol Oncol. 2021. PMID: 34821131 Free PMC article. Review.
-
Post-translational modifications on the retinoblastoma protein.J Biomed Sci. 2022 Jun 1;29(1):33. doi: 10.1186/s12929-022-00818-x. J Biomed Sci. 2022. PMID: 35650644 Free PMC article. Review.
-
Large-Scale Assessment of Bioinformatics Tools for Lysine Succinylation Sites.Cells. 2019 Jan 28;8(2):95. doi: 10.3390/cells8020095. Cells. 2019. PMID: 30696115 Free PMC article. Review.
-
The non-specific lethal (NSL) complex at the crossroads of transcriptional control and cellular homeostasis.EMBO Rep. 2019 Jul;20(7):e47630. doi: 10.15252/embr.201847630. Epub 2019 Jun 3. EMBO Rep. 2019. PMID: 31267707 Free PMC article. Review.
References
-
- Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 - PubMed
-
- Strahl B. D., Allis C. D. (2000) The language of covalent histone modifications. Nature 403, 41–45 - PubMed
-
- Starai V. J., Celic I., Cole R. N., Boeke J. D., Escalante-Semerena J. C. (2002) Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298, 2390–2392 - PubMed
-
- Hebert A. S., Dittenhafer-Reed K. E., Yu W., Bailey D. J., Selen E. S., Boersma M. D., Carson J. J., Tonelli M., Balloon A. J., Higbee A. J., Westphall M. S., Pagliarini D. J., Prolla T. A., Assadi-Porter F., Roy S., Denu J. M., Coon J. J. (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

