A baculovirus-mediated strategy for full-length plant virus coat protein expression and purification
- PMID: 23945471
- PMCID: PMC3765376
- DOI: 10.1186/1743-422X-10-262
A baculovirus-mediated strategy for full-length plant virus coat protein expression and purification
Abstract
Background: Garlic production is severely affected by virus infection, causing a decrease in productivity and quality. There are no virus-free cultivars and garlic-infecting viruses are difficult to purify, which make specific antibody production very laborious. Since high quality antisera against plant viruses are important tools for serological detection, we have developed a method to express and purify full-length plant virus coat proteins using baculovirus expression system and insects as bioreactors.
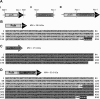
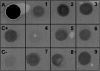
Results: In this work, we have fused the full-length coat protein (cp) gene from the Garlic Mite-borne Filamentous Virus (GarMbFV) to the 3'-end of the Polyhedrin (polh) gene of the baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV). The recombinant baculovirus was amplified in insect cell culture and the virus was used to infect Spodoptera frugiperda larvae. Thus, the recombinant fused protein was easily purified from insect cadavers using sucrose gradient centrifugation and analyzed by Western Blotting. Interestingly, amorphous crystals were produced in the cytoplasm of cells infected with the recombinant virus containing the chimeric-protein gene but not in cells infected with the wild type and recombinant virus containing the hexa histidine tagged Polh. Moreover, the chimeric protein was used to immunize rats and generate antibodies against the target protein. The antiserum produced was able to detect plants infected with GarMbFV, which had been initially confirmed by RT-PCR.
Conclusions: The expression of a plant virus full-length coat protein fused to the baculovirus Polyhedrin in recombinant baculovirus-infected insects was shown to produce high amounts of the recombinant protein which was easily purified and efficiently used to generate specific antibodies. Therefore, this strategy can potentially be used for the development of plant virus diagnostic kits for those viruses that are difficult to purify, are present in low titers or are present in mix infection in their plant hosts.
Figures
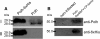



Similar articles
-
Recombinant expression of Garlic virus C (GARV-C) capsid protein in insect cells and its potential for the production of specific antibodies.Microbiol Res. 2008;163(3):354-61. doi: 10.1016/j.micres.2006.06.016. Epub 2006 Aug 4. Microbiol Res. 2008. PMID: 16890415
-
N-terminal in coat protein of Garlic virus X is indispensible for its serological detection.Virus Genes. 2014 Feb;48(1):128-32. doi: 10.1007/s11262-013-0990-3. Epub 2013 Oct 18. Virus Genes. 2014. PMID: 24136255
-
Production and purification of VP2 protein of porcine parvovirus expressed in an insect-baculovirus cell system.Virol J. 2010 Dec 10;7:366. doi: 10.1186/1743-422X-7-366. Virol J. 2010. PMID: 21143963 Free PMC article.
-
Easily purified baculovirus/insect-system-expressed recombinant hepatitis B virus surface antigen fused to the N- or C-terminus of polyhedrin.Arch Virol. 2022 Feb;167(2):345-354. doi: 10.1007/s00705-021-05305-6. Epub 2021 Nov 28. Arch Virol. 2022. PMID: 34839419
-
Questions surrounding the taxonomic validity of the species Garlic mite-borne filamentous virus (genus Allexivirus).Arch Virol. 2019 Sep;164(9):2367-2370. doi: 10.1007/s00705-019-04333-7. Epub 2019 Jun 29. Arch Virol. 2019. PMID: 31256263 Review.
Cited by
-
Complete genome sequence of the first non-Asian isolate of Bombyx mori nucleopolyhedrovirus.Virus Genes. 2014 Dec;49(3):477-84. doi: 10.1007/s11262-014-1112-6. Epub 2014 Sep 12. Virus Genes. 2014. PMID: 25212430
-
Expression of tomato yellow leaf curl virus coat protein using baculovirus expression system and evaluation of its utility as a viral antigen.3 Biotech. 2017 Aug;7(4):269. doi: 10.1007/s13205-017-0893-4. Epub 2017 Jul 29. 3 Biotech. 2017. PMID: 28794924 Free PMC article.
-
A new theraphosid spider toxin causes early insect cell death by necrosis when expressed in vitro during recombinant baculovirus infection.PLoS One. 2013 Dec 13;8(12):e84404. doi: 10.1371/journal.pone.0084404. eCollection 2013. PLoS One. 2013. PMID: 24349574 Free PMC article.
-
A Chikungunya Virus Multiepitope Recombinant Protein Expressed from the Binary System Insect Cell/Recombinant Baculovirus Is Useful for Laboratorial Diagnosis of Chikungunya.Microorganisms. 2022 Jul 18;10(7):1451. doi: 10.3390/microorganisms10071451. Microorganisms. 2022. PMID: 35889170 Free PMC article.
-
A new virus found in garlic virus complex is a member of possible novel genus of the family Betaflexiviridae (order Tymovirales).PeerJ. 2019 Jan 16;7:e6285. doi: 10.7717/peerj.6285. eCollection 2019. PeerJ. 2019. PMID: 30671312 Free PMC article.
References
-
- Lot H, Chovelon V, Souche S, Delecolle B. Effects of onion yellow dwarf and leek yellow stripe viruses on symptomatology and yield loss of three French garlic cultivars. Plant Dis. 1998;10:5. - PubMed
-
- Conci VC, Canavelli A, Lunello P, Rienzo JD, Nome SF, Zumelzu G, Italia R. Yield losses associated with virus-infected garlic plants during five successive years. Plant Dis. 2003;10:5. - PubMed
-
- Lunello P, Rienzo JD, Conci VC. Yield Loss in Garlic Caused by Leek yellow stripe virus Argentinean Isolate. Plant Dis. 2007;10:6. - PubMed
-
- Torres AC, Fajardo TV, Dusi AN, Resende RO, Buso JA. Shoot tip culture and thermotherapy for recovering virus-free plants of garlic. Horticultura Brasileira. 2000;10:3.
-
- Tsuneyoshi T, Sumi S. Differentiation among garlic viruses in mixed infections based on RT-PCR procedures and direct tissue blotting immunoassays. Phytopathology. 1996;10:7.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

