Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis
- PMID: 23940516
- PMCID: PMC3734136
- DOI: 10.1371/journal.pone.0069182
Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis
Abstract
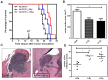
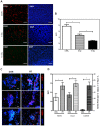
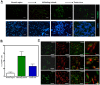
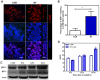
Radiation therapy (RT) remains the front-line treatment for high-grade gliomas; however, tumor recurrence remains the main obstacle for the clinical success of RT. Using a murine astrocytoma tumor cell line, ALTS1C1, the present study demonstrates that whole brain irradiation prolonged the survival of tumor-bearing mice, although the mice eventually died associated with increased tumor infiltration. Immunohistochemical (IHC) analysis indicated that RT decreased the microvascular density (MVD) of the primary tumor core, but increased the MVD of the tumor invasion front. RT also increased the number of tumor-associated macrophages (TAMs) and the expression of stromal cell-derived factor-1 (SDF-1) and hypoxia-inducible factor-1 (HIF-1) at the tumor invasion front. SDF-1 expression suppressed by siRNA (SDFkd tumors) showed a decrease in RT-enhanced tumor invasiveness, leading to prolonged survival of mice bearing these tumors. The invasion front in SDFkd tumors showed a lower MVD and TAM density than that in the islands of the control or irradiated ALTS1C1 tumors. Our results indicate that tumor-secreted SDF-1 is one key factor in RT-induced tumor invasiveness, and that it exerts its effect likely through macrophage mobilization and tumor revascularization.
Conflict of interest statement
Figures
Similar articles
-
Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model.Lab Invest. 2012 Jan;92(1):151-62. doi: 10.1038/labinvest.2011.128. Epub 2011 Sep 5. Lab Invest. 2012. PMID: 21894147
-
Effects of pre-irradiation and SDF-1 suppression on the progression of murine astrocytoma cells grown in different stromal beds.Int J Radiat Biol. 2014 Dec;90(12):1162-8. doi: 10.3109/09553002.2014.930539. Epub 2014 Jul 7. Int J Radiat Biol. 2014. PMID: 24937369
-
Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12.Brain. 2006 Sep;129(Pt 9):2426-35. doi: 10.1093/brain/awl173. Epub 2006 Jul 10. Brain. 2006. PMID: 16835250
-
Radiation Damage to Tumor Vasculature Initiates a Program That Promotes Tumor Recurrences.Int J Radiat Oncol Biol Phys. 2020 Nov 1;108(3):734-744. doi: 10.1016/j.ijrobp.2020.05.028. Epub 2020 May 28. Int J Radiat Oncol Biol Phys. 2020. PMID: 32473180 Review.
-
Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy.Br J Radiol. 2014 Mar;87(1035):20130686. doi: 10.1259/bjr.20130686. Br J Radiol. 2014. PMID: 24338942 Free PMC article. Review.
Cited by
-
Probing tumor microenvironment in patients with newly diagnosed glioblastoma during chemoradiation and adjuvant temozolomide with functional MRI.Sci Rep. 2018 Nov 20;8(1):17062. doi: 10.1038/s41598-018-34820-x. Sci Rep. 2018. PMID: 30459364 Free PMC article.
-
Effects of radiation on the metastatic process.Mol Med. 2018 Apr 24;24(1):16. doi: 10.1186/s10020-018-0015-8. Mol Med. 2018. PMID: 30134800 Free PMC article. Review.
-
M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma.Oncotarget. 2017 Aug 7;8(42):72597-72612. doi: 10.18632/oncotarget.19994. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 29069812 Free PMC article.
-
STAT3 Contributes to Radioresistance in Cancer.Front Oncol. 2020 Jul 7;10:1120. doi: 10.3389/fonc.2020.01120. eCollection 2020. Front Oncol. 2020. PMID: 32733808 Free PMC article. Review.
-
Tumor Microenvironment in Glioma Invasion.Brain Sci. 2022 Apr 15;12(4):505. doi: 10.3390/brainsci12040505. Brain Sci. 2022. PMID: 35448036 Free PMC article. Review.
References
-
- Louis DN (2006) Molecular pathology of malignant gliomas. Annu Rev Pathol 1: 97–117. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996. - PubMed
-
- Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP (1993) Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 71: 2585–2597. - PubMed
-
- Canazza A, Calatozzolo C, Fumagalli L, Bergantin A, Ghielmetti F, et al. (2011) Increased migration of a human glioma cell line after in vitro CyberKnife irradiation. Cancer Biol Ther 12: 629–633. - PubMed
-
- Wild-Bode C, Weller M, Rimner A, Dichgans J, Wick W (2001) Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 61: 2744–2750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

