Conditional knockout of tumor overexpressed gene in mouse neurons affects RNA granule assembly, granule translation, LTP and short term habituation
- PMID: 23936366
- PMCID: PMC3735573
- DOI: 10.1371/journal.pone.0069989
Conditional knockout of tumor overexpressed gene in mouse neurons affects RNA granule assembly, granule translation, LTP and short term habituation
Abstract
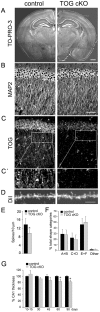
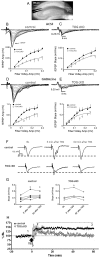
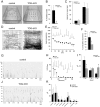
In neurons, specific RNAs are assembled into granules, which are translated in dendrites, however the functional consequences of granule assembly are not known. Tumor overexpressed gene (TOG) is a granule-associated protein containing multiple binding sites for heterogeneous nuclear ribonucleoprotein (hnRNP) A2, another granule component that recognizes cis-acting sequences called hnRNP A2 response elements (A2REs) present in several granule RNAs. Translation in granules is sporadic, which is believed to reflect monosomal translation, with occasional bursts, which are believed to reflect polysomal translation. In this study, TOG expression was conditionally knocked out (TOG cKO) in mouse hippocampal neurons using cre/lox technology. In TOG cKO cultured neurons granule assembly and bursty translation of activity-regulated cytoskeletal associated (ARC) mRNA, an A2RE RNA, are disrupted. In TOG cKO brain slices synaptic sensitivity and long term potentiation (LTP) are reduced. TOG cKO mice exhibit hyperactivity, perseveration and impaired short term habituation. These results suggest that in hippocampal neurons TOG is required for granule assembly, granule translation and synaptic plasticity, and affects behavior.
Conflict of interest statement
Figures


Similar articles
-
Multivalent Molecules as Modulators of RNA Granule Size and Composition.Biophys J. 2017 Jul 25;113(2):235-245. doi: 10.1016/j.bpj.2017.01.031. Epub 2017 Feb 24. Biophys J. 2017. PMID: 28242011 Free PMC article.
-
The microtubule-associated protein tumor overexpressed gene binds to the RNA trafficking protein heterogeneous nuclear ribonucleoprotein A2.Mol Biol Cell. 2005 Apr;16(4):1938-47. doi: 10.1091/mbc.e04-08-0709. Epub 2005 Feb 9. Mol Biol Cell. 2005. PMID: 15703215 Free PMC article.
-
The microtubule-associated protein tumor overexpressed gene/cytoskeleton-associated protein 5 is necessary for myelin basic protein expression in oligodendrocytes.J Neurosci. 2007 Jul 18;27(29):7654-62. doi: 10.1523/JNEUROSCI.0203-07.2007. J Neurosci. 2007. PMID: 17634360 Free PMC article.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
Cited by
-
Multivalent Molecules as Modulators of RNA Granule Size and Composition.Biophys J. 2017 Jul 25;113(2):235-245. doi: 10.1016/j.bpj.2017.01.031. Epub 2017 Feb 24. Biophys J. 2017. PMID: 28242011 Free PMC article.
-
Conditional Knockout of Cav2.1 Disrupts the Accuracy of Spatial Recognition of CA1 Place Cells and Spatial/Contextual Recognition Behavior.Front Behav Neurosci. 2016 Nov 3;10:214. doi: 10.3389/fnbeh.2016.00214. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27857685 Free PMC article.
-
Development of single-molecule ubiquitination mediated fluorescence complementation to visualize protein ubiquitination dynamics in dendrites.Cell Rep. 2022 Nov 15;41(7):111658. doi: 10.1016/j.celrep.2022.111658. Cell Rep. 2022. PMID: 36384114 Free PMC article.
-
Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis?Semin Cell Dev Biol. 2016 Nov;59:141-156. doi: 10.1016/j.semcdb.2016.01.003. Epub 2016 Jan 15. Semin Cell Dev Biol. 2016. PMID: 26779951 Free PMC article. Review.
-
Conditional knockout of TOG results in CNS hypomyelination.Glia. 2017 Mar;65(3):489-501. doi: 10.1002/glia.23106. Epub 2017 Jan 7. Glia. 2017. PMID: 28063167 Free PMC article.
References
-
- Al-Bassam J, Larsen NA, Hyman AA, Harrison SC (2007) Crystal structure of a TOG domain: Conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 15: 355–362. - PubMed
-
- Charrasse S, Schroeder M, Gauthier-Rouviere C, Ango F, Cassimeris L, et al. (1998) The TOGp protein is a new human microtubule-associated protein homologous to the xenopus XMAP215. J Cell Sci 111 (Pt 10): 1371–1383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

