Distinct SUMO ligases cooperate with Esc2 and Slx5 to suppress duplication-mediated genome rearrangements
- PMID: 23935535
- PMCID: PMC3731205
- DOI: 10.1371/journal.pgen.1003670
Distinct SUMO ligases cooperate with Esc2 and Slx5 to suppress duplication-mediated genome rearrangements
Erratum in
-
Correction: Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements.PLoS Genet. 2016 Aug 31;12(8):e1006302. doi: 10.1371/journal.pgen.1006302. eCollection 2016 Aug. PLoS Genet. 2016. PMID: 27579875 Free PMC article.
Abstract
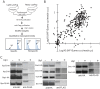
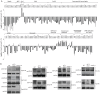
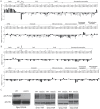
Suppression of duplication-mediated gross chromosomal rearrangements (GCRs) is essential to maintain genome integrity in eukaryotes. Here we report that SUMO ligase Mms21 has a strong role in suppressing GCRs in Saccharomyces cerevisiae, while Siz1 and Siz2 have weaker and partially redundant roles. Understanding the functions of these enzymes has been hampered by a paucity of knowledge of their substrate specificity in vivo. Using a new quantitative SUMO-proteomics technology, we found that Siz1 and Siz2 redundantly control the abundances of most sumoylated substrates, while Mms21 more specifically regulates sumoylation of RNA polymerase-I and the SMC-family proteins. Interestingly, Esc2, a SUMO-like domain-containing protein, specifically promotes the accumulation of sumoylated Mms21-specific substrates and functions with Mms21 to suppress GCRs. On the other hand, the Slx5-Slx8 complex, a SUMO-targeted ubiquitin ligase, suppresses the accumulation of sumoylated Mms21-specific substrates. Thus, distinct SUMO ligases work in concert with Esc2 and Slx5-Slx8 to control substrate specificity and sumoylation homeostasis to prevent GCRs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Shared and distinct roles of Esc2 and Mms21 in suppressing genome rearrangements and regulating intracellular sumoylation.PLoS One. 2021 Feb 18;16(2):e0247132. doi: 10.1371/journal.pone.0247132. eCollection 2021. PLoS One. 2021. PMID: 33600463 Free PMC article.
-
Activation of the Slx5-Slx8 ubiquitin ligase by poly-small ubiquitin-like modifier conjugates.J Biol Chem. 2008 Jul 18;283(29):19912-21. doi: 10.1074/jbc.M802690200. Epub 2008 May 22. J Biol Chem. 2008. PMID: 18499666 Free PMC article.
-
Multiple domains in Siz SUMO ligases contribute to substrate selectivity.J Cell Sci. 2006 Nov 15;119(Pt 22):4749-57. doi: 10.1242/jcs.03243. Epub 2006 Oct 31. J Cell Sci. 2006. PMID: 17077124
-
SUMO-targeted ubiquitin ligases.Biochim Biophys Acta. 2014 Jan;1843(1):75-85. doi: 10.1016/j.bbamcr.2013.08.022. Epub 2013 Sep 7. Biochim Biophys Acta. 2014. PMID: 24018209 Review.
-
Genome maintenance in Saccharomyces cerevisiae: the role of SUMO and SUMO-targeted ubiquitin ligases.Nucleic Acids Res. 2017 Mar 17;45(5):2242-2261. doi: 10.1093/nar/gkw1369. Nucleic Acids Res. 2017. PMID: 28115630 Free PMC article. Review.
Cited by
-
PolySUMOylation by Siz2 and Mms21 triggers relocation of DNA breaks to nuclear pores through the Slx5/Slx8 STUbL.Genes Dev. 2016 Apr 15;30(8):931-45. doi: 10.1101/gad.277665.116. Epub 2016 Apr 7. Genes Dev. 2016. PMID: 27056668 Free PMC article.
-
A SUMO-dependent pathway controls elongating RNA Polymerase II upon UV-induced damage.Sci Rep. 2019 Nov 29;9(1):17914. doi: 10.1038/s41598-019-54027-y. Sci Rep. 2019. PMID: 31784551 Free PMC article.
-
Non-Smc element 5 (Nse5) of the Smc5/6 complex interacts with SUMO pathway components.Biol Open. 2016 Jun 15;5(6):777-85. doi: 10.1242/bio.018440. Biol Open. 2016. PMID: 27215325 Free PMC article.
-
SUMO orchestrates multiple alternative DNA-protein crosslink repair pathways.Cell Rep. 2021 Nov 23;37(8):110034. doi: 10.1016/j.celrep.2021.110034. Cell Rep. 2021. PMID: 34818558 Free PMC article.
-
The role of SUMOylation in biomolecular condensate dynamics and protein localization.Cell Insight. 2024 Sep 10;3(6):100199. doi: 10.1016/j.cellin.2024.100199. eCollection 2024 Dec. Cell Insight. 2024. PMID: 39399482 Free PMC article. Review.
References
-
- Gordenin DA, Resnick MA (1998) Yeast ARMs (DNA at-risk motifs) can reveal sources of genome instability. Mutat Res 400: 45–58. - PubMed
-
- Deininger PL, Batzer MA (1999) Alu repeats and human disease. Mol Genet Metab 67: 183–193 doi:10.1006/mgme.1999.2864 - DOI - PubMed
-
- Ji Y, Eichler EE, Schwartz S, Nicholls RD (2000) Structure of chromosomal duplicons and their role in mediating human genomic disorders. Genome Res 10: 597–610. - PubMed
-
- Kolodner RD, Putnam CD, Myung K (2002) Maintenance of genome stability in Saccharomyces cerevisiae. Science 297: 552–557 doi:10.1126/science.1075277 - DOI - PubMed
-
- Putnam CD, Hayes TK, Kolodner RD (2009) Specific pathways prevent duplication-mediated genome rearrangements. Nature 460: 984–989 doi:10.1038/nature08217 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

