IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity
- PMID: 23935505
- PMCID: PMC3731249
- DOI: 10.1371/journal.ppat.1003531
IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity
Abstract
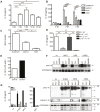
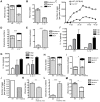
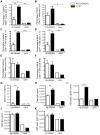
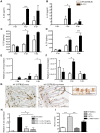
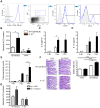
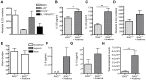
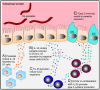
Approximately 2 billion people currently suffer from intestinal helminth infections, which are typically chronic in nature and result in growth retardation, vitamin A deficiency, anemia and poor cognitive function. Such chronicity results from co-evolution between helminths and their mammalian hosts; however, the molecular mechanisms by which these organisms avert immune rejection are not clear. We have found that the natural murine helminth, Heligmosomoides polygyrus bakeri (Hp) elicits the secretion of IL-1β in vivo and in vitro and that this cytokine is critical for shaping a mucosal environment suited to helminth chronicity. Indeed in mice deficient for IL-1β (IL-1β(-/-)), or treated with the soluble IL-1βR antagonist, Anakinra, helminth infection results in enhanced type 2 immunity and accelerated parasite expulsion. IL-1β acts to decrease production of IL-25 and IL-33 at early time points following infection and parasite rejection was determined to require IL-25. Taken together, these data indicate that Hp promotes the release of host-derived IL-1β that suppresses the release of innate cytokines, resulting in suboptimal type 2 immunity and allowing pathogen chronicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Concerted IL-25R and IL-4Rα signaling drive innate type 2 effector immunity for optimal helminth expulsion.Elife. 2018 Sep 21;7:e38269. doi: 10.7554/eLife.38269. Elife. 2018. PMID: 30238872 Free PMC article.
-
The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus.J Immunol. 2003 Jan 1;170(1):384-93. doi: 10.4049/jimmunol.170.1.384. J Immunol. 2003. PMID: 12496423
-
Critical Role for Interleukin-25 in Host Protective Th2 Memory Response against Heligmosomoides polygyrus bakeri.Infect Immun. 2016 Nov 18;84(12):3328-3337. doi: 10.1128/IAI.00180-16. Print 2016 Dec. Infect Immun. 2016. PMID: 27620722 Free PMC article.
-
Immunity to the model intestinal helminth parasite Heligmosomoides polygyrus.Semin Immunopathol. 2012 Nov;34(6):829-46. doi: 10.1007/s00281-012-0347-3. Epub 2012 Oct 11. Semin Immunopathol. 2012. PMID: 23053394 Free PMC article. Review.
-
Regulation of type 2 immunity to helminths by mast cells.Gut Microbes. 2012 Sep-Oct;3(5):476-81. doi: 10.4161/gmic.21507. Epub 2012 Aug 15. Gut Microbes. 2012. PMID: 22892692 Free PMC article. Review.
Cited by
-
Trained immunity of intestinal tuft cells during infancy enhances host defense against enteroviral infections in mice.EMBO Mol Med. 2024 Oct;16(10):2516-2538. doi: 10.1038/s44321-024-00128-9. Epub 2024 Sep 11. EMBO Mol Med. 2024. PMID: 39261649 Free PMC article.
-
The Potential Nexus between Helminths and SARS-CoV-2 Infection: A Literature Review.J Immunol Res. 2023 Jun 20;2023:5544819. doi: 10.1155/2023/5544819. eCollection 2023. J Immunol Res. 2023. PMID: 37383608 Free PMC article. Review.
-
Microbial Components and Effector Molecules in T Helper Cell Differentiation and Function.Immune Netw. 2023 Feb 22;23(1):e7. doi: 10.4110/in.2023.23.e7. eCollection 2023 Feb. Immune Netw. 2023. PMID: 36911805 Free PMC article. Review.
-
The Immune Response to Nematode Infection.Int J Mol Sci. 2023 Jan 23;24(3):2283. doi: 10.3390/ijms24032283. Int J Mol Sci. 2023. PMID: 36768605 Free PMC article. Review.
-
Fasciola hepatica primoinfections and reinfections in sheep drive distinct Th1/Th2/Treg immune responses in liver and hepatic lymph node at early and late stages.Vet Res. 2023 Jan 10;54(1):2. doi: 10.1186/s13567-022-01129-7. Vet Res. 2023. PMID: 36627694 Free PMC article.
References
-
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, et al. (2003) Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19: 547–551. - PubMed
-
- WHO (2010) Working to overcome the global impact of neglected tropical diseases, First WHO report on Neglected Tropical Diseases. - PubMed
-
- Monroy FG, Enriquez FJ (1992) Heligmosomoides polygyrus: a model for chronic gastrointestinal helminthiasis. Parasitol Today 8: 49–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

