The viral chemokine MCK-2 of murine cytomegalovirus promotes infection as part of a gH/gL/MCK-2 complex
- PMID: 23935483
- PMCID: PMC3723581
- DOI: 10.1371/journal.ppat.1003493
The viral chemokine MCK-2 of murine cytomegalovirus promotes infection as part of a gH/gL/MCK-2 complex
Abstract
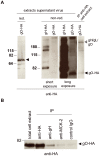
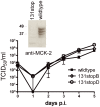
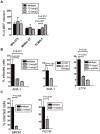
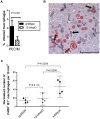
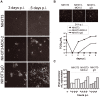
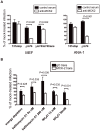
Human cytomegalovirus (HCMV) forms two gH/gL glycoprotein complexes, gH/gL/gO and gH/gL/pUL(128,130,131A), which determine the tropism, the entry pathways and the mode of spread of the virus. For murine cytomegalovirus (MCMV), which serves as a model for HCMV, a gH/gL/gO complex functionally homologous to the HCMV gH/gL/gO complex has been described. Knock-out of MCMV gO does impair, but not abolish, virus spread indicating that also MCMV might form an alternative gH/gL complex. Here, we show that the MCMV CC chemokine MCK-2 forms a complex with the glycoprotein gH, a complex which is incorporated into the virion. We could additionally show that mutants lacking both, gO and MCK-2 are not able to produce infectious virus. Trans-complementation of these double mutants with either gO or MCK-2 showed that both proteins can promote infection of host cells, although through different entry pathways. MCK-2 has been extensively studied in vivo by others. It has been shown to be involved in attracting cells for virus dissemination and in regulating antiviral host responses. We now show that MCK-2, by forming a complex with gH, strongly promotes infection of macrophages in vitro and in vivo. Thus, MCK-2 may play a dual role in MCMV infection, as a chemokine regulating the host response and attracting specific target cells and as part of a glycoprotein complex promoting entry into cells crucial for virus dissemination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Murine Cytomegalovirus MCK-2 Facilitates In Vivo Infection Transfer from Dendritic Cells to Salivary Gland Acinar Cells.J Virol. 2021 Aug 10;95(17):e0069321. doi: 10.1128/JVI.00693-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34132572 Free PMC article.
-
Mouse Cytomegalovirus Differentially Exploits Cell Surface Glycosaminoglycans in a Cell Type-Dependent and MCK-2-Independent Manner.Viruses. 2019 Dec 27;12(1):31. doi: 10.3390/v12010031. Viruses. 2019. PMID: 31892128 Free PMC article.
-
Principles for studying in vivo attenuation of virus mutants: defining the role of the cytomegalovirus gH/gL/gO complex as a paradigm.Med Microbiol Immunol. 2015 Jun;204(3):295-305. doi: 10.1007/s00430-015-0405-2. Epub 2015 Mar 18. Med Microbiol Immunol. 2015. PMID: 25782576 Review.
-
Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry.PLoS Pathog. 2017 Apr 12;13(4):e1006281. doi: 10.1371/journal.ppat.1006281. eCollection 2017 Apr. PLoS Pathog. 2017. PMID: 28403202 Free PMC article.
-
Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism.Viruses. 2018 Dec 11;10(12):704. doi: 10.3390/v10120704. Viruses. 2018. PMID: 30544948 Free PMC article. Review.
Cited by
-
Chemokines and phosphatidylserine: New binding partners for apoptotic cell clearance.Front Cell Dev Biol. 2022 Aug 25;10:943590. doi: 10.3389/fcell.2022.943590. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092729 Free PMC article. No abstract available.
-
Anticytomegalovirus Peptides Point to New Insights for CMV Entry Mechanisms and the Limitations of In Vitro Screenings.mSphere. 2019 Feb 13;4(1):e00586-18. doi: 10.1128/mSphere.00586-18. mSphere. 2019. PMID: 30760613 Free PMC article.
-
Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus.PLoS Pathog. 2017 Aug 30;13(8):e1006601. doi: 10.1371/journal.ppat.1006601. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28854233 Free PMC article.
-
Mutual Interference between Cytomegalovirus and Reconstitution of Protective Immunity after Hematopoietic Cell Transplantation.Front Immunol. 2016 Aug 4;7:294. doi: 10.3389/fimmu.2016.00294. eCollection 2016. Front Immunol. 2016. PMID: 27540380 Free PMC article. Review.
-
Immune surveillance of cytomegalovirus in tissues.Cell Mol Immunol. 2024 Sep;21(9):959-981. doi: 10.1038/s41423-024-01186-2. Epub 2024 Aug 12. Cell Mol Immunol. 2024. PMID: 39134803 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

