Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission
- PMID: 23921378
- PMCID: PMC3779755
- DOI: 10.1074/jbc.M113.479873
Adaptor proteins MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and are specific for mitochondrial fission
Abstract
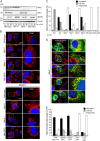
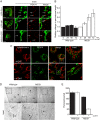
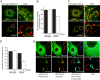
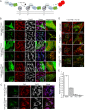
Drp1 (dynamin-related protein 1) is recruited to both mitochondrial and peroxisomal membranes to execute fission. Fis1 and Mff are Drp1 receptor/effector proteins of mitochondria and peroxisomes. Recently, MiD49 and MiD51 were also shown to recruit Drp1 to the mitochondrial surface; however, different reports have ascribed opposing roles in fission and fusion. Here, we show that MiD49 or MiD51 overexpression blocked fission by acting in a dominant-negative manner by sequestering Drp1 specifically at mitochondria, causing unopposed fusion events at mitochondria along with elongation of peroxisomes. Mitochondrial elongation caused by MiD49/51 overexpression required the action of fusion mediators mitofusins 1 and 2. Furthermore, at low level overexpression when MiD49 and MiD51 form discrete foci at mitochondria, mitochondrial fission events still occurred. Unlike Fis1 and Mff, MiD49 and MiD51 were not targeted to the peroxisomal surface, suggesting that they specifically act to facilitate Drp1-directed fission at mitochondria. Moreover, when MiD49 or MiD51 was targeted to the surface of peroxisomes or lysosomes, Drp1 was specifically recruited to these organelles. Moreover, the Drp1 recruitment activity of MiD49/51 appeared stronger than that of Mff or Fis1. We conclude that MiD49 and MiD51 can act independently of Mff and Fis1 in Drp1 recruitment and suggest that they provide specificity to the division of mitochondria.
Keywords: Cell Biology; Confocal Microscopy; Drp1; Fission; Mammal; MiD49; MiD51; Mitochondria; Morphology; Peroxisomes.
Figures

Similar articles
-
Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission.Mol Biol Cell. 2013 Mar;24(5):659-67. doi: 10.1091/mbc.E12-10-0721. Epub 2013 Jan 2. Mol Biol Cell. 2013. PMID: 23283981 Free PMC article.
-
The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease.Clin Sci (Lond). 2016 Nov 1;130(21):1861-74. doi: 10.1042/CS20160030. Clin Sci (Lond). 2016. PMID: 27660309 Review.
-
Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission.J Cell Sci. 2016 Jun 1;129(11):2170-81. doi: 10.1242/jcs.185165. Epub 2016 Apr 12. J Cell Sci. 2016. PMID: 27076521 Free PMC article.
-
Drp1, Mff, Fis1, and MiD51 are coordinated to mediate mitochondrial fission during UV irradiation-induced apoptosis.FASEB J. 2016 Jan;30(1):466-76. doi: 10.1096/fj.15-274258. Epub 2015 Oct 2. FASEB J. 2016. PMID: 26432782
-
The Drp1-Mediated Mitochondrial Fission Protein Interactome as an Emerging Core Player in Mitochondrial Dynamics and Cardiovascular Disease Therapy.Int J Mol Sci. 2023 Mar 17;24(6):5785. doi: 10.3390/ijms24065785. Int J Mol Sci. 2023. PMID: 36982862 Free PMC article. Review.
Cited by
-
Unique fractal evaluation and therapeutic implications of mitochondrial morphology in malignant mesothelioma.Sci Rep. 2016 Apr 15;6:24578. doi: 10.1038/srep24578. Sci Rep. 2016. PMID: 27080907 Free PMC article.
-
Uncovering the important role of mitochondrial dynamics in oogenesis: impact on fertility and metabolic disorder transmission.Biophys Rev. 2021 Nov 23;13(6):967-981. doi: 10.1007/s12551-021-00891-w. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35059021 Free PMC article. Review.
-
Apogossypol-mediated reorganisation of the endoplasmic reticulum antagonises mitochondrial fission and apoptosis.Cell Death Dis. 2019 Jul 8;10(7):521. doi: 10.1038/s41419-019-1759-y. Cell Death Dis. 2019. PMID: 31285422 Free PMC article.
-
The mitochondrial fission receptor MiD51 requires ADP as a cofactor.Structure. 2014 Mar 4;22(3):367-77. doi: 10.1016/j.str.2014.01.001. Epub 2014 Feb 6. Structure. 2014. PMID: 24508339 Free PMC article.
-
Epigenetic Dysregulation of the Dynamin-Related Protein 1 Binding Partners MiD49 and MiD51 Increases Mitotic Mitochondrial Fission and Promotes Pulmonary Arterial Hypertension: Mechanistic and Therapeutic Implications.Circulation. 2018 Jul 17;138(3):287-304. doi: 10.1161/CIRCULATIONAHA.117.031258. Epub 2018 Feb 5. Circulation. 2018. PMID: 29431643 Free PMC article.
References
-
- Elgass K., Pakay J., Ryan M. T., Palmer C. S. (2013) Recent advances into the understanding of mitochondrial fission. Biochim. Biophys. Acta 1833, 150–161 - PubMed
-
- Westermann B. (2010) Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872–884 - PubMed
-
- Chan D. C. (2012) Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 - PubMed
-
- Li Z., Okamoto K., Hayashi Y., Sheng M. (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873–887 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

