Type 2 alveolar cells are stem cells in adult lung
- PMID: 23921127
- PMCID: PMC3696553
- DOI: 10.1172/JCI68782
Type 2 alveolar cells are stem cells in adult lung
Abstract
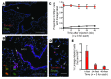
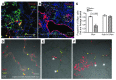
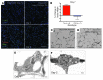
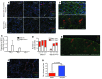
Gas exchange in the lung occurs within alveoli, air-filled sacs composed of type 2 and type 1 epithelial cells (AEC2s and AEC1s), capillaries, and various resident mesenchymal cells. Here, we use a combination of in vivo clonal lineage analysis, different injury/repair systems, and in vitro culture of purified cell populations to obtain new information about the contribution of AEC2s to alveolar maintenance and repair. Genetic lineage-tracing experiments showed that surfactant protein C-positive (SFTPC-positive) AEC2s self renew and differentiate over about a year, consistent with the population containing long-term alveolar stem cells. Moreover, if many AEC2s were specifically ablated, high-resolution imaging of intact lungs showed that individual survivors undergo rapid clonal expansion and daughter cell dispersal. Individual lineage-labeled AEC2s placed into 3D culture gave rise to self-renewing "alveolospheres," which contained both AEC2s and cells expressing multiple AEC1 markers, including HOPX, a new marker for AEC1s. Growth and differentiation of the alveolospheres occurred most readily when cocultured with primary PDGFRα⁺ lung stromal cells. This population included lipofibroblasts that normally reside close to AEC2s and may therefore contribute to a stem cell niche in the murine lung. Results suggest that a similar dynamic exists between AEC2s and mesenchymal cells in the human lung.
Figures
Similar articles
-
The Oxygen Environment at Birth Specifies the Population of Alveolar Epithelial Stem Cells in the Adult Lung.Stem Cells. 2016 May;34(5):1396-406. doi: 10.1002/stem.2330. Epub 2016 Mar 7. Stem Cells. 2016. PMID: 26891117 Free PMC article.
-
TAZ is required for lung alveolar epithelial cell differentiation after injury.JCI Insight. 2019 Jun 18;5(14):e128674. doi: 10.1172/jci.insight.128674. JCI Insight. 2019. PMID: 31211697 Free PMC article.
-
CTGF promotes the repair and regeneration of alveoli after acute lung injury by promoting the proliferation of subpopulation of AEC2s.Respir Res. 2023 Sep 23;24(1):227. doi: 10.1186/s12931-023-02512-4. Respir Res. 2023. PMID: 37741976 Free PMC article.
-
Regulation of alveolar type 2 stem/progenitor cells in lung injury and regeneration.Acta Biochim Biophys Sin (Shanghai). 2020 Jul 10;52(7):716-722. doi: 10.1093/abbs/gmaa052. Acta Biochim Biophys Sin (Shanghai). 2020. PMID: 32445469 Review.
-
Niche Cells and Signals that Regulate Lung Alveolar Stem Cells In Vivo.Cold Spring Harb Perspect Biol. 2020 Dec 1;12(12):a035717. doi: 10.1101/cshperspect.a035717. Cold Spring Harb Perspect Biol. 2020. PMID: 32179507 Free PMC article. Review.
Cited by
-
State of the art on lung organoids in mammals.Vet Res. 2021 Jun 2;52(1):77. doi: 10.1186/s13567-021-00946-6. Vet Res. 2021. PMID: 34078444 Free PMC article. Review.
-
Imaging lung regeneration by light sheet microscopy.Histochem Cell Biol. 2021 Feb;155(2):271-277. doi: 10.1007/s00418-020-01903-8. Epub 2020 Jul 19. Histochem Cell Biol. 2021. PMID: 32685990 Free PMC article.
-
Decoy ACE2-expressing extracellular vesicles that competitively bind SARS-CoV-2 as a possible COVID-19 therapy.Clin Sci (Lond). 2020 Jun 26;134(12):1301-1304. doi: 10.1042/CS20200623. Clin Sci (Lond). 2020. PMID: 32542396 Free PMC article.
-
An Optimized Method to Isolate Human Fibroblasts from Tissue for ex vivo Analysis.Bio Protoc. 2019 Dec 5;9(23):e3440. doi: 10.21769/BioProtoc.3440. eCollection 2019 Dec 5. Bio Protoc. 2019. PMID: 33654935 Free PMC article.
-
You Say You Want a Resolution (of Fibrosis).Am J Respir Cell Mol Biol. 2020 Oct;63(4):424-435. doi: 10.1165/rcmb.2020-0182TR. Am J Respir Cell Mol Biol. 2020. PMID: 32640171 Free PMC article. Review.
References
-
- Kauffman SL. Cell proliferation in the mammalian lung. Int Rev Exp Pathol. 1980;22:131–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

