Linking environmental variability to village-scale malaria transmission using a simple immunity model
- PMID: 23919581
- PMCID: PMC3750354
- DOI: 10.1186/1756-3305-6-226
Linking environmental variability to village-scale malaria transmission using a simple immunity model
Abstract
Background: Individuals continuously exposed to malaria gradually acquire immunity that protects from severe disease and high levels of parasitization. Acquired immunity has been incorporated into numerous models of malaria transmission of varying levels of complexity (e.g. Bull World Health Organ 50:347, 1974; Am J Trop Med Hyg 75:19, 2006; Math Biosci 90:385-396, 1988). Most such models require prescribing inputs of mosquito biting rates or other entomological or epidemiological information. Here, we present a model with a novel structure that uses environmental controls of mosquito population dynamics to simulate the mosquito biting rates, malaria prevalence as well as variability in protective immunity of the population.
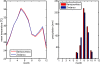
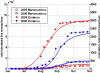
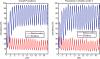
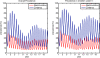
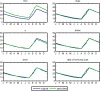
Methods: A simple model of acquired immunity to malaria is presented and tested within the framework of the Hydrology, Entomology and Malaria Transmission Simulator (HYDREMATS), a coupled hydrology and agent-based entomology model. The combined model uses environmental data including rainfall, temperature, and topography to simulate malaria prevalence and level of acquired immunity in the human population. The model is used to demonstrate the effect of acquired immunity on malaria prevalence in two Niger villages that are hydrologically and entomologically very different. Simulations are conducted for the year 2006 and compared to malaria prevalence observations collected from the two villages.
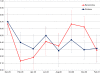
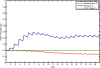
Results: Blood smear samples from children show no clear difference in malaria prevalence between the two villages despite pronounced differences in observed mosquito abundance. The similarity in prevalence is attributed to the moderating effect of acquired immunity, which depends on prior exposure to the parasite through infectious bites - and thus the hydrologically determined mosquito abundance. Modelling the level of acquired immunity can affect village vulnerability to climatic anomalies.
Conclusions: The model presented has a novel structure constituting a mechanistic link between spatial and temporal environmental variability and village-scale malaria transmission. Incorporating acquired immunity into the model has allowed simulation of prevalence in the two villages, and isolation of the effects of acquired immunity in dampening the difference in prevalence between the two villages. Without these effects, the difference in prevalence between the two villages would have been significantly larger in response to the large differences in mosquito populations and the associated biting rates.
Figures


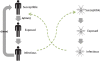
Similar articles
-
A mechanistic approach for accurate simulation of village scale malaria transmission.Malar J. 2009 Oct 2;8:223. doi: 10.1186/1475-2875-8-223. Malar J. 2009. PMID: 19799793 Free PMC article.
-
Early warnings of the potential for malaria transmission in rural Africa using the hydrology, entomology and malaria transmission simulator (HYDREMATS).Malar J. 2010 Nov 12;9:323. doi: 10.1186/1475-2875-9-323. Malar J. 2010. PMID: 21073726 Free PMC article.
-
Seasonal malaria vector and transmission dynamics in western Burkina Faso.Malar J. 2019 Apr 2;18(1):113. doi: 10.1186/s12936-019-2747-5. Malar J. 2019. PMID: 30940141 Free PMC article.
-
El Niño and associated outbreaks of severe malaria in highland populations in Irian Jaya, Indonesia: a review and epidemiological perspective.Southeast Asian J Trop Med Public Health. 1999 Dec;30(4):608-19. Southeast Asian J Trop Med Public Health. 1999. PMID: 10928348 Review.
-
Acquired immunity to malaria.Clin Microbiol Rev. 2009 Jan;22(1):13-36, Table of Contents. doi: 10.1128/CMR.00025-08. Clin Microbiol Rev. 2009. PMID: 19136431 Free PMC article. Review.
Cited by
-
Environmental Determinants of Malaria Transmission Around the Koka Reservoir in Ethiopia.Geohealth. 2018 Mar 30;2(3):104-115. doi: 10.1002/2017GH000108. eCollection 2018 Mar. Geohealth. 2018. PMID: 32159012 Free PMC article.
-
Agent-based models of malaria transmission: a systematic review.Malar J. 2018 Aug 17;17(1):299. doi: 10.1186/s12936-018-2442-y. Malar J. 2018. PMID: 30119664 Free PMC article. Review.
-
Mathematical modeling of climate change and malaria transmission dynamics: a historical review.J Math Biol. 2018 Oct;77(4):857-933. doi: 10.1007/s00285-018-1229-7. Epub 2018 Apr 24. J Math Biol. 2018. PMID: 29691632 Review.
-
malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication.PLoS Med. 2017 Nov 30;14(11):e1002452. doi: 10.1371/journal.pmed.1002452. eCollection 2017 Nov. PLoS Med. 2017. PMID: 29190279 Free PMC article. Review.
-
A validated agent-based model to study the spatial and temporal heterogeneities of malaria incidence in the rainforest environment.Malar J. 2015 Dec 22;14:514. doi: 10.1186/s12936-015-1030-7. Malar J. 2015. PMID: 26696294 Free PMC article.
References
-
- Molineaux L, Muir D, Spencer H, Wernsdorfer W, McGregor I. The epidemiology of malaria and its measurement. Malaria: Princ Pract Malariol. 1988;2:999–1089.
-
- Agnandji ST. et al.First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365(20):1863–1875. - PubMed
-
- Smith T, Maire N, Dietz K, Killeen GF, Vounatsou P, Molineaux L, Tanner M. Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;75(2 suppl):11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

