Cardiomyocyte-specific p65 NF-κB deletion protects the injured heart by preservation of calcium handling
- PMID: 23913709
- PMCID: PMC3798748
- DOI: 10.1152/ajpheart.00067.2013
Cardiomyocyte-specific p65 NF-κB deletion protects the injured heart by preservation of calcium handling
Abstract
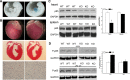
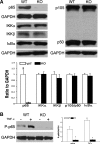
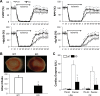
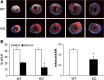
NF-κB is a well-known transcription factor that is intimately involved with inflammation and immunity. We have previously shown that NF-κB promotes inflammatory events and mediates adverse cardiac remodeling following ischemia reperfusion (I/R). Conversely, others have pointed to the beneficial influence of NF-κB in I/R injury related to its anti-apoptotic effects. Understanding the seemingly disparate influence of manipulating NF-κB is hindered, in part, by current approaches that only indirectly interfere with the function of its most transcriptionally active unit, p65 NF-κB. Mice were generated with cardiomyocyte-specific deletion of p65 NF-κB. Phenotypically, these mice and their hearts appeared normal. Basal and stimulated p65 expression were significantly reduced in whole hearts and completely ablated in isolated cardiomyocytes. When compared with wild-type mice, transgenic animals were protected from both global I/R by Langendorff as well as regional I/R by coronary ligation and release. The protected, transgenic hearts had less cytokine activity and decreased apoptosis. Furthermore, p65 ablation was associated with enhanced calcium reuptake by the sarcoplasmic reticulum. This influence on calcium handling was related to increased expression of phosphorylated phospholamban in conditional p65 null mice. In conclusion, cardiomyocyte-specific deletion of the most active, canonical NF-κB subunit affords cardioprotection to both global and regional I/R injury. The beneficial effects of NF-κB inhibition are related, in part, to modulation of intracellular calcium homeostasis.
Keywords: calcium; ischemia-reperfusion; nuclear factor-κB; phospholamban.
Figures
Similar articles
-
Ca2+/Calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB.Circ Res. 2013 Mar 15;112(6):935-44. doi: 10.1161/CIRCRESAHA.112.276915. Epub 2013 Feb 6. Circ Res. 2013. PMID: 23388157 Free PMC article.
-
Runx1 Deficiency Protects Against Adverse Cardiac Remodeling After Myocardial Infarction.Circulation. 2018 Jan 2;137(1):57-70. doi: 10.1161/CIRCULATIONAHA.117.028911. Epub 2017 Oct 13. Circulation. 2018. PMID: 29030345 Free PMC article.
-
Novel role of HAX-1 in ischemic injury protection involvement of heat shock protein 90.Circ Res. 2013 Jan 4;112(1):79-89. doi: 10.1161/CIRCRESAHA.112.279935. Epub 2012 Sep 14. Circ Res. 2013. PMID: 22982986 Free PMC article.
-
Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium infarction in hearts with Ca2+/calmodulin kinase II constitutive phosphorylation of ryanodine receptors.Cardiovasc Res. 2019 Mar 1;115(3):556-569. doi: 10.1093/cvr/cvy213. Cardiovasc Res. 2019. PMID: 30169578 Free PMC article.
-
Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure.Cardiovasc Res. 2011 Jan 1;89(1):129-38. doi: 10.1093/cvr/cvq274. Epub 2010 Aug 25. Cardiovasc Res. 2011. PMID: 20797985 Free PMC article.
Cited by
-
NFκB promotes oxidative stress-induced necrosis and ischemia/reperfusion injury by inhibiting Nrf2-ARE pathway.Free Radic Biol Med. 2020 Nov 1;159:125-135. doi: 10.1016/j.freeradbiomed.2020.07.031. Epub 2020 Jul 31. Free Radic Biol Med. 2020. PMID: 32745764 Free PMC article.
-
RELA governs a network of islet-specific metabolic genes necessary for beta cell function.Diabetologia. 2023 Aug;66(8):1516-1531. doi: 10.1007/s00125-023-05931-6. Epub 2023 Jun 14. Diabetologia. 2023. PMID: 37311878 Free PMC article.
-
Severe T-System Remodeling in Pediatric Viral Myocarditis.Front Cardiovasc Med. 2021 Jan 18;7:624776. doi: 10.3389/fcvm.2020.624776. eCollection 2020. Front Cardiovasc Med. 2021. PMID: 33537349 Free PMC article.
-
Inflammation in Myocardial Ischemia/Reperfusion Injury: Underlying Mechanisms and Therapeutic Potential.Antioxidants (Basel). 2023 Oct 31;12(11):1944. doi: 10.3390/antiox12111944. Antioxidants (Basel). 2023. PMID: 38001797 Free PMC article. Review.
-
Ischemic postconditioning reduced myocardial ischemia-reperfusion injury: The roles of melatonin and uncoupling protein 3.Anatol J Cardiol. 2020 Jan;23(1):19-27. doi: 10.14744/AnatolJCardiol.2019.72609. Anatol J Cardiol. 2020. PMID: 31911566 Free PMC article.
References
-
- Aksoy MO, Bin W, Yang Y, Yun-You D, Kelsen SG. Nuclear factor-κB augments β2-adrenergic receptor expression in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L1271–L1278, 2001 - PubMed
-
- Barry WH, Zhang XQ, Halkos ME, Vinten-Johansen J, Saegusa N, Spitzer KW, Matsuoka N, Sheets M, Rao NV, Kennedy TP. Nonanticoagulant heparin reduces myocyte Na+ and Ca2+ loading during simulated ischemia and decreases reperfusion injury. Am J Physiol Heart Circ Physiol 298: H102–H111, 2010 - PMC - PubMed
-
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376: 167–170, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

