The composition, functions, and regulation of the budding yeast kinetochore
- PMID: 23908374
- PMCID: PMC3730914
- DOI: 10.1534/genetics.112.145276
The composition, functions, and regulation of the budding yeast kinetochore
Abstract
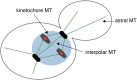
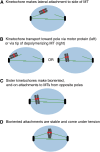
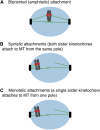
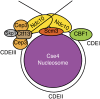
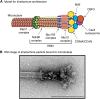
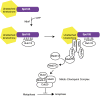
The propagation of all organisms depends on the accurate and orderly segregation of chromosomes in mitosis and meiosis. Budding yeast has long served as an outstanding model organism to identify the components and underlying mechanisms that regulate chromosome segregation. This review focuses on the kinetochore, the macromolecular protein complex that assembles on centromeric chromatin and maintains persistent load-bearing attachments to the dynamic tips of spindle microtubules. The kinetochore also serves as a regulatory hub for the spindle checkpoint, ensuring that cell cycle progression is coupled to the achievement of proper microtubule-kinetochore attachments. Progress in understanding the composition and overall architecture of the kinetochore, as well as its properties in making and regulating microtubule attachments and the spindle checkpoint, is discussed.
Keywords: biorientation; budding yeast; kinetochore; microtubules; spindle checkpoint.
Figures

Similar articles
-
Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast.J Cell Biol. 2004 Feb 16;164(4):535-46. doi: 10.1083/jcb.200308100. Epub 2004 Feb 9. J Cell Biol. 2004. PMID: 14769859 Free PMC article.
-
Chromosomal attachments set length and microtubule number in the Saccharomyces cerevisiae mitotic spindle.Mol Biol Cell. 2014 Dec 15;25(25):4034-48. doi: 10.1091/mbc.E14-01-0016. Epub 2014 Oct 15. Mol Biol Cell. 2014. PMID: 25318669 Free PMC article.
-
Roles for the conserved spc105p/kre28p complex in kinetochore-microtubule binding and the spindle assembly checkpoint.PLoS One. 2009 Oct 28;4(10):e7640. doi: 10.1371/journal.pone.0007640. PLoS One. 2009. PMID: 19893618 Free PMC article.
-
Kinetochore composition and its function: lessons from yeasts.FEMS Microbiol Rev. 2014 Mar;38(2):185-200. doi: 10.1111/1574-6976.12049. FEMS Microbiol Rev. 2014. PMID: 24666101 Review.
-
Kinetochore-microtubule error correction for biorientation: lessons from yeast.Biochem Soc Trans. 2024 Feb 28;52(1):29-39. doi: 10.1042/BST20221261. Biochem Soc Trans. 2024. PMID: 38305688 Free PMC article. Review.
Cited by
-
Structural characterization of KKT4, an unconventional microtubule-binding kinetochore protein.Structure. 2021 Sep 2;29(9):1014-1028.e8. doi: 10.1016/j.str.2021.04.004. Epub 2021 Apr 28. Structure. 2021. PMID: 33915106 Free PMC article.
-
Mck1-mediated proteolysis of CENP-A prevents mislocalization of CENP-A for chromosomal stability in Saccharomyces cerevisiae.Genetics. 2024 Sep 4;228(1):iyae108. doi: 10.1093/genetics/iyae108. Genetics. 2024. PMID: 38984710 Free PMC article.
-
A case of convergent-gene interference in the budding yeast knockout library causing chromosome instability.G3 (Bethesda). 2021 May 7;11(5):jkab084. doi: 10.1093/g3journal/jkab084. G3 (Bethesda). 2021. PMID: 33724427 Free PMC article.
-
Shaping centromeres to resist mitotic spindle forces.J Cell Sci. 2022 Feb 15;135(4):jcs259532. doi: 10.1242/jcs.259532. Epub 2022 Feb 18. J Cell Sci. 2022. PMID: 35179192 Free PMC article.
-
ISW1a modulates cohesin distribution in centromeric and pericentromeric regions.Nucleic Acids Res. 2023 Sep 22;51(17):9101-9121. doi: 10.1093/nar/gkad612. Nucleic Acids Res. 2023. PMID: 37486771 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

