Dystonin/BPAG1 promotes plus-end-directed transport of herpes simplex virus 1 capsids on microtubules during entry
- PMID: 23903849
- PMCID: PMC3807277
- DOI: 10.1128/JVI.01633-13
Dystonin/BPAG1 promotes plus-end-directed transport of herpes simplex virus 1 capsids on microtubules during entry
Abstract
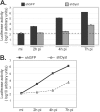
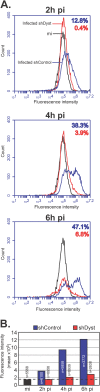
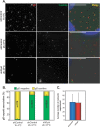
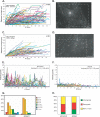
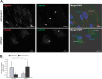
During infection by herpes simplex virus 1 (HSV-1), the viral capsid is transported around the cytoplasm along the microtubule (MT) network. Although molecular motors have been implicated in this process, the composition of the molecular machinery required for efficient directional transport is unknown. We previously showed that dystonin (BPAG1) is recruited to HSV-1 capsids by the capsid-bound tegument protein pUL37 to promote efficient cytoplasmic transport of capsids during egress. Dystonin is a cytoskeleton cross-linker which localizes at MT plus ends and has roles in retrograde and anterograde transport in neurons. In this study, we investigated the role of dystonin during the entry stages of HSV-1 infection. Because of the way in which the MT network is organized, capsids are required to change their direction of motion along the MTs as they travel from the point of entry to the nucleus, where replication takes place. Thus, capsids first travel to the centrosome (the principal microtubule organizing center) by minus-end-directed transport and then switch polarity and travel to the nucleus by plus-end-directed transport. We observed that transport of capsids toward the centrosome was slowed, but not blocked, by dystonin depletion. However, transport of capsids away from the centrosome was significantly impaired, causing them to accumulate in the vicinity of the centrosome and reducing the numbers reaching the nucleus. We conclude that, during entry of HSV-1, dystonin has a specific role in plus-ended transport of capsids from the centrosome to the nucleus.
Figures

Similar articles
-
Herpesvirus tegument protein pUL37 interacts with dystonin/BPAG1 to promote capsid transport on microtubules during egress.J Virol. 2013 Mar;87(5):2857-67. doi: 10.1128/JVI.02676-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269794 Free PMC article.
-
Differing effects of herpes simplex virus 1 and pseudorabies virus infections on centrosomal function.J Virol. 2013 Jun;87(12):7102-12. doi: 10.1128/JVI.00764-13. Epub 2013 Apr 17. J Virol. 2013. PMID: 23596303 Free PMC article.
-
Function of dynein and dynactin in herpes simplex virus capsid transport.Mol Biol Cell. 2002 Aug;13(8):2795-809. doi: 10.1091/mbc.01-07-0348. Mol Biol Cell. 2002. PMID: 12181347 Free PMC article.
-
HIV-1 capsid exploitation of the host microtubule cytoskeleton during early infection.Retrovirology. 2021 Jul 6;18(1):19. doi: 10.1186/s12977-021-00563-3. Retrovirology. 2021. PMID: 34229718 Free PMC article. Review.
-
HSV-1 Cytoplasmic Envelopment and Egress.Int J Mol Sci. 2020 Aug 19;21(17):5969. doi: 10.3390/ijms21175969. Int J Mol Sci. 2020. PMID: 32825127 Free PMC article. Review.
Cited by
-
BPAG1a and b associate with EB1 and EB3 and modulate vesicular transport, Golgi apparatus structure, and cell migration in C2.7 myoblasts.PLoS One. 2014 Sep 22;9(9):e107535. doi: 10.1371/journal.pone.0107535. eCollection 2014. PLoS One. 2014. PMID: 25244344 Free PMC article.
-
Susceptibility loci revealed for bovine respiratory disease complex in pre-weaned holstein calves.BMC Genomics. 2014 Dec 22;15(1):1164. doi: 10.1186/1471-2164-15-1164. BMC Genomics. 2014. PMID: 25534905 Free PMC article.
-
Spectraplakin family proteins - cytoskeletal crosslinkers with versatile roles.J Cell Sci. 2017 Aug 1;130(15):2447-2457. doi: 10.1242/jcs.196154. Epub 2017 Jul 5. J Cell Sci. 2017. PMID: 28679697 Free PMC article. Review.
-
Insights into herpesvirus tegument organization from structural analyses of the 970 central residues of HSV-1 UL36 protein.J Biol Chem. 2015 Apr 3;290(14):8820-33. doi: 10.1074/jbc.M114.612838. Epub 2015 Feb 12. J Biol Chem. 2015. PMID: 25678705 Free PMC article.
-
Microtubule-Dependent Trafficking of Alphaherpesviruses in the Nervous System: The Ins and Outs.Viruses. 2019 Dec 17;11(12):1165. doi: 10.3390/v11121165. Viruses. 2019. PMID: 31861082 Free PMC article. Review.
References
-
- Bartolini F, Gundersen GG. 2006. Generation of noncentrosomal microtubule arrays. J. Cell Sci. 119:4155–4163. - PubMed
-
- Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, Karger A, Sodeik B. 2010. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 6:e1000991. 10.1371/journal.ppat.1000991. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

