Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson's disease
- PMID: 23902940
- PMCID: PMC3781407
- DOI: 10.1124/jpet.113.207639
Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson's disease
Abstract
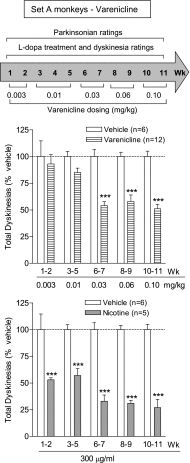
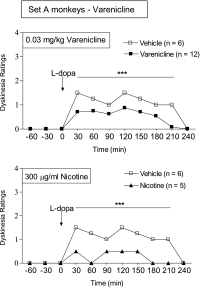
Abnormal involuntary movements or dyskinesias are a serious complication of long-term l-DOPA treatment of Parkinson's disease, for which there are few treatment options. Accumulating preclinical data show that nicotine decreases l-DOPA-induced dyskinesias (LIDs), suggesting that it may be a useful antidyskinetic therapy for Parkinson's disease. Here, we investigated whether nicotinic acetylcholine receptor (nAChR) agonists reduced LIDs in nonhuman primates. We first tested the nonselective nAChR agonist 1, 6,7,8,9-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine (varenicline), which offers the advantage that it is approved by the U.S. Food and Drug Administration for use in humans. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned monkeys (n = 23) were first administered l-DOPA/carbidopa (10/2.5 mg/kg) twice daily 5 days/week until stably dyskinetic. Oral varenicline (0.03-0.10 mg/kg) decreased LIDs ∼50% compared with vehicle-treated monkeys, whereas nicotine treatment (300 µg/ml in drinking water) reduced LIDs by 70% in a parallel group of animals. We next tested the selective α4β2*/α6β2* nAChR agonist TC-8831 [3-cyclopropylcarbonyl-3,6-diazabicyclo[3.1.1]heptane] on LIDs in the same set of monkeys after a 10-week washout. We also tested TC-8831 in another set of MPTP-lesioned monkeys (n = 16) that were nAChR drug-naïve. Oral TC-8831 (0.03-0.3 mg/kg) reduced LIDs in both sets by 30-50%. After a washout period, repeat TC-8831 dosing led to a greater decline in LIDs (60%) in both sets of monkeys that was similar to the effect of nicotine. Tolerance to any nAChR drug did not develop over the course of the study (3-4 months). NAChR drug treatment did not worsen parkinsonism or cognitive ability. These data suggest that nAChR agonists may be useful for the management of dyskinesias in l-DOPA-treated Parkinson's disease patients.
Figures


Similar articles
-
Alpha7 nicotinic receptors as therapeutic targets for Parkinson's disease.Biochem Pharmacol. 2015 Oct 15;97(4):399-407. doi: 10.1016/j.bcp.2015.06.014. Epub 2015 Jun 18. Biochem Pharmacol. 2015. PMID: 26093062 Free PMC article. Review.
-
ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson's disease.Mov Disord. 2014 Apr;29(4):508-17. doi: 10.1002/mds.25817. Epub 2014 Feb 11. Mov Disord. 2014. PMID: 24515328 Free PMC article.
-
The α7 nicotinic receptor agonist ABT-107 decreases L-Dopa-induced dyskinesias in parkinsonian monkeys.J Pharmacol Exp Ther. 2014 Oct;351(1):25-32. doi: 10.1124/jpet.114.216283. Epub 2014 Jul 17. J Pharmacol Exp Ther. 2014. PMID: 25034405 Free PMC article.
-
Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson's disease.Mov Disord. 2013 Sep;28(10):1398-406. doi: 10.1002/mds.25594. Epub 2013 Jul 8. Mov Disord. 2013. PMID: 23836409 Free PMC article.
-
Role for the nicotinic cholinergic system in movement disorders; therapeutic implications.Pharmacol Ther. 2014 Oct;144(1):50-9. doi: 10.1016/j.pharmthera.2014.05.004. Epub 2014 May 14. Pharmacol Ther. 2014. PMID: 24836728 Free PMC article. Review.
Cited by
-
Evidence for a role for α6(∗) nAChRs in l-dopa-induced dyskinesias using Parkinsonian α6(∗) nAChR gain-of-function mice.Neuroscience. 2015 Jun 4;295:187-97. doi: 10.1016/j.neuroscience.2015.03.040. Epub 2015 Mar 24. Neuroscience. 2015. PMID: 25813704 Free PMC article.
-
α7 nicotinic receptor agonists reduce levodopa-induced dyskinesias with severe nigrostriatal damage.Mov Disord. 2015 Dec;30(14):1901-1911. doi: 10.1002/mds.26453. Epub 2015 Nov 17. Mov Disord. 2015. PMID: 26573698 Free PMC article.
-
Alpha7 nicotinic receptors as therapeutic targets for Parkinson's disease.Biochem Pharmacol. 2015 Oct 15;97(4):399-407. doi: 10.1016/j.bcp.2015.06.014. Epub 2015 Jun 18. Biochem Pharmacol. 2015. PMID: 26093062 Free PMC article. Review.
-
In vitro and in vivo neuronal nicotinic receptor properties of (+)- and (-)-pyrido[3,4]homotropane [(+)- and (-)-PHT]: (+)-PHT is a potent and selective full agonist at α6β2 containing neuronal nicotinic acetylcholine receptors.ACS Chem Neurosci. 2015 Jun 17;6(6):920-6. doi: 10.1021/acschemneuro.5b00077. Epub 2015 Apr 30. ACS Chem Neurosci. 2015. PMID: 25891987 Free PMC article.
-
ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson's disease.Mov Disord. 2014 Apr;29(4):508-17. doi: 10.1002/mds.25817. Epub 2014 Feb 11. Mov Disord. 2014. PMID: 24515328 Free PMC article.
References
-
- Bordia T, Campos C, Huang L, Quik M. (2008) Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther 327:239–247 - PubMed
-
- Brotchie J, Jenner P. (2011) New approaches to therapy. Int Rev Neurobiol 98:123–150 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
