Class D β-lactamases: a reappraisal after five decades
- PMID: 23902256
- PMCID: PMC4018812
- DOI: 10.1021/ar300327a
Class D β-lactamases: a reappraisal after five decades
Abstract
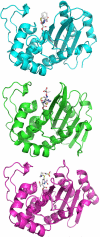
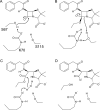
Despite 70 years of clinical use, β-lactam antibiotics still remain at the forefront of antimicrobial chemotherapy. The major challenge to these life-saving therapeutics is the presence of bacterial enzymes (i.e., β-lactamases) that can hydrolyze the β-lactam bond and inactivate the antibiotic. These enzymes can be grouped into four classes (A-D). Among the most genetically diverse are the class D β-lactamases. In this class are β-lactamases that can inactivate the entire spectrum of β-lactam antibiotics (penicillins, cephalosporins, and carbapenems). Class D β-lactamases are mostly found in Gram-negative bacteria such as Pseudomonas aeruginosa , Escherichia coli , Proteus mirabilis , and Acinetobacter baumannii . The active-sites of class D β-lactamases contain an unusual N-carboxylated lysine post-translational modification. A strongly hydrophobic active-site helps create the conditions that allow the lysine to combine with CO2, and the resulting carbamate is stabilized by a number of hydrogen bonds. The carboxy-lysine plays a symmetric role in the reaction, serving as a general base to activate the serine nucleophile in the acylation reaction, and the deacylating water in the second step. There are more than 250 class D β-lactamases described, and the full set of variants shows remarkable diversity with regard to substrate binding and turnover. Narrow-spectrum variants are most effective against the earliest generation penicillins and cephalosporins such as ampicillin and cephalothin. Extended-spectrum variants (also known as extended-spectrum β-lactamases, ESBLs) pose a more dangerous clinical threat as they possess a small number of substitutions that allow them to bind and hydrolyze later generation cephalosporins that contain bulkier side-chain constituents (e.g., cefotaxime, ceftazidime, and cefepime). Mutations that permit this versatility seem to cluster in the area surrounding an active-site tryptophan resulting in a widened active-site to accommodate the oxyimino side-chains of these cephalosporins. More concerning are the class D β-lactamases that hydrolyze clinically important carbapenem β-lactam drugs (e.g., imipenem). Whereas carbapenems irreversibly acylate and inhibit narrow-spectrum β-lactamases, class D carbapenemases are able to recruit and activate a deacylating water. The rotational orientation of the C6 hydroxyethyl group found on all carbapenem antibiotics likely plays a role in whether the deacylating water is effective or not. Inhibition of class D β-lactamases is a current challenge. Commercially available inhibitors that are active against other classes of β-lactamases are ineffective against class D enzymes. On the horizon are several compounds, consisting of both β-lactam derivatives and non-β-lactams, that have the potential of providing novel leads to design new mechanism-based inactivators that are effective against the class D enzymes. Several act synergistically when given in combination with a β-lactam antibiotic, and others show a unique mechanism of inhibition that is distinct from the traditional β-lactamase inhibitors. These studies will bolster structure-based inhibitor design efforts to facilitate the optimization and development of these compounds as class D inactivators.
Figures

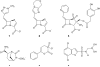
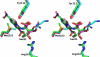
Similar articles
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
β-Lactamases and β-Lactamase Inhibitors in the 21st Century.J Mol Biol. 2019 Aug 23;431(18):3472-3500. doi: 10.1016/j.jmb.2019.04.002. Epub 2019 Apr 5. J Mol Biol. 2019. PMID: 30959050 Free PMC article. Review.
-
[Mechanisms of resistance in Enterobacteriaceae towards beta-lactamase antibiotics].Acta Med Croatica. 2004;58(4):307-12. Acta Med Croatica. 2004. PMID: 15700687 Croatian.
-
Inhibition of the class C beta-lactamase from Acinetobacter spp.: insights into effective inhibitor design.Biochemistry. 2010 Jan 19;49(2):329-40. doi: 10.1021/bi9015988. Biochemistry. 2010. PMID: 19925018 Free PMC article.
-
Structure of ADC-68, a novel carbapenem-hydrolyzing class C extended-spectrum β-lactamase isolated from Acinetobacter baumannii.Acta Crystallogr D Biol Crystallogr. 2014 Nov;70(Pt 11):2924-36. doi: 10.1107/S1399004714019543. Epub 2014 Oct 23. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 25372683
Cited by
-
Metagenomic insights into the microbial community structure and resistomes of a tropical agricultural soil persistently inundated with pesticide and animal manure use.Folia Microbiol (Praha). 2022 Oct;67(5):707-719. doi: 10.1007/s12223-022-00970-9. Epub 2022 Apr 12. Folia Microbiol (Praha). 2022. PMID: 35415828
-
Theaflavin-3,3´-digallate increases the antibacterial activity of β-lactam antibiotics by inhibiting metallo-β-lactamase activity.J Cell Mol Med. 2019 Oct;23(10):6955-6964. doi: 10.1111/jcmm.14580. Epub 2019 Aug 8. J Cell Mol Med. 2019. PMID: 31392792 Free PMC article.
-
Arginine Modulates Carbapenem Deactivation by OXA-24/40 in Acinetobacter baumannii.J Mol Biol. 2021 Sep 17;433(19):167150. doi: 10.1016/j.jmb.2021.167150. Epub 2021 Jul 14. J Mol Biol. 2021. PMID: 34271009 Free PMC article.
-
Metallo-β-lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design.Chem Rev. 2021 Jul 14;121(13):7957-8094. doi: 10.1021/acs.chemrev.1c00138. Epub 2021 Jun 15. Chem Rev. 2021. PMID: 34129337 Free PMC article. Review.
-
Structural and Biochemical Features of OXA-517: a Carbapenem and Expanded-Spectrum Cephalosporin Hydrolyzing OXA-48 Variant.Antimicrob Agents Chemother. 2023 Feb 16;67(2):e0109522. doi: 10.1128/aac.01095-22. Epub 2023 Jan 17. Antimicrob Agents Chemother. 2023. PMID: 36648230 Free PMC article.
References
-
- McDermott W, Rogers DE. Social ramifications of control of microbial disease. Johns Hopkins Med. J. 1982;151:302–12. - PubMed
-
- Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, Ecker DJ, Massire C, Eshoo MW, Sampath R, Thomson JM, Rather PN, Craft DW, Fishbain JT, Ewell AJ, Jacobs MR, Paterson DL, Bonomo RA. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 2006;50:4114–23. - PMC - PubMed
-
- Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob. Chemother. 2012;67:1597–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

