Virtual memory CD8 T cells display unique functional properties
- PMID: 23898211
- PMCID: PMC3746847
- DOI: 10.1073/pnas.1307572110
Virtual memory CD8 T cells display unique functional properties
Abstract
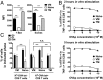
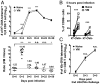
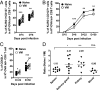
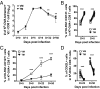
Previous studies revealed the existence of foreign antigen-specific memory phenotype CD8 T cells in unimmunized mice. Considerable evidence suggests this population, termed "virtual memory" (VM) CD8 T cells, arise via physiological homeostatic mechanisms. However, the antigen-specific function of VM cells is poorly characterized, and hence their potential contribution to immune responses against pathogens is unclear. Here we show that naturally occurring, polyclonal VM cells have unique functional properties, distinct from either naïve or antigen-primed memory CD8 T cells. In striking contrast to conventional memory cells, VM cells showed poor T cell receptor-induced IFN-γ synthesis and preferentially differentiated into central memory phenotype cells after priming. Importantly, VM cells showed efficient control of Listeria monocytogenes infection, indicating memory-like capacity to eliminate certain pathogens. These data suggest naturally arising VM cells display unique functional traits, allowing them to form a bridge between the innate and adaptive phase of a response to pathogens.
Keywords: homeostasis; lymphocyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Simultaneous assessment of antigen-stimulated cytokine production and memory subset composition of memory CD8 T cells.J Immunol Methods. 2006 Jun 30;313(1-2):161-8. doi: 10.1016/j.jim.2006.04.005. Epub 2006 May 26. J Immunol Methods. 2006. PMID: 16762359
-
Progesterone impairs antigen-non-specific immune protection by CD8 T memory cells via interferon-γ gene hypermethylation.PLoS Pathog. 2017 Nov 20;13(11):e1006736. doi: 10.1371/journal.ppat.1006736. eCollection 2017 Nov. PLoS Pathog. 2017. PMID: 29155896 Free PMC article.
-
A role for IFN-gamma from antigen-specific CD8+ T cells in protective immunity to Listeria monocytogenes.J Immunol. 2007 Aug 15;179(4):2457-66. doi: 10.4049/jimmunol.179.4.2457. J Immunol. 2007. PMID: 17675507
-
Listeria monocytogenes: a model pathogen to study antigen-specific memory CD8 T cell responses.Semin Immunopathol. 2015 May;37(3):301-10. doi: 10.1007/s00281-015-0477-5. Epub 2015 Apr 10. Semin Immunopathol. 2015. PMID: 25860798 Free PMC article. Review.
-
Hiding in Plain Sight: Virtually Unrecognizable Memory Phenotype CD8+ T cells.Int J Mol Sci. 2020 Nov 16;21(22):8626. doi: 10.3390/ijms21228626. Int J Mol Sci. 2020. PMID: 33207648 Free PMC article. Review.
Cited by
-
Cell States and Interactions of CD8 T Cells and Disease-Enriched Microglia in Human Brains with Alzheimer's Disease.Biomedicines. 2024 Jan 29;12(2):308. doi: 10.3390/biomedicines12020308. Biomedicines. 2024. PMID: 38397909 Free PMC article.
-
IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection.J Exp Med. 2016 Jun 27;213(7):1319-29. doi: 10.1084/jem.20151359. Epub 2016 Jun 13. J Exp Med. 2016. PMID: 27298446 Free PMC article.
-
Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner.Nat Commun. 2016 Apr 21;7:11291. doi: 10.1038/ncomms11291. Nat Commun. 2016. PMID: 27097762 Free PMC article.
-
Immune Predictors of Mortality After Ribonucleic Acid Virus Infection.J Infect Dis. 2020 Mar 2;221(6):882-889. doi: 10.1093/infdis/jiz531. J Infect Dis. 2020. PMID: 31621854 Free PMC article.
-
SATB1 ensures appropriate transcriptional programs within naïve CD8+ T cells.Immunol Cell Biol. 2022 Sep;100(8):636-652. doi: 10.1111/imcb.12566. Epub 2022 Jul 13. Immunol Cell Biol. 2022. PMID: 35713361 Free PMC article.
References
-
- Jameson SC. T cell homeostasis: Keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17(3):231–237. - PubMed
-
- Marleau AM, Sarvetnick N. T cell homeostasis in tolerance and immunity. J Leukoc Biol. 2005;78(3):575–584. - PubMed
-
- Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17(3):183–191. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

