An empirical approach towards the efficient and optimal production of influenza-neutralizing ovine polyclonal antibodies demonstrates that the novel adjuvant CoVaccine HT™ is functionally superior to Freund's adjuvant
- PMID: 23894371
- PMCID: PMC3720891
- DOI: 10.1371/journal.pone.0068895
An empirical approach towards the efficient and optimal production of influenza-neutralizing ovine polyclonal antibodies demonstrates that the novel adjuvant CoVaccine HT™ is functionally superior to Freund's adjuvant
Abstract
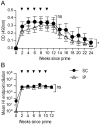
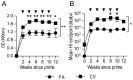
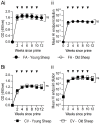
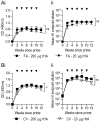
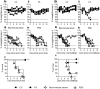
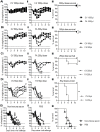
Passive immunotherapies utilising polyclonal antibodies could have a valuable role in preventing and treating infectious diseases such as influenza, particularly in pandemic situations but also in immunocompromised populations such as the elderly, the chronically immunosuppressed, pregnant women, infants and those with chronic diseases. The aim of this study was to optimise current methods used to generate ovine polyclonal antibodies. Polyclonal antibodies to baculovirus-expressed recombinant influenza haemagglutinin from A/Puerto Rico/8/1934 H1N1 (PR8) were elicited in sheep using various immunisation regimens designed to investigate the priming immunisation route, adjuvant formulation, sheep age, and antigen dose, and to empirically ascertain which combination maximised antibody output. The novel adjuvant CoVaccine HT™ was compared to Freund's adjuvant which is currently the adjuvant of choice for commercial production of ovine polyclonal Fab therapies. CoVaccine HT™ induced significantly higher titres of functional ovine anti-haemagglutinin IgG than Freund's adjuvant but with fewer side effects, including reduced site reactions. Polyclonal hyperimmune sheep sera effectively neutralised influenza virus in vitro and, when given before or after influenza virus challenge, prevented the death of infected mice. Neither the age of the sheep nor the route of antigen administration appeared to influence antibody titre. Moreover, reducing the administrated dose of haemagglutinin antigen minimally affected antibody titre. Together, these results suggest a cost effective way of producing high and sustained yields of functional ovine polyclonal antibodies specifically for the prevention and treatment of globally significant diseases.
Conflict of interest statement
Figures

Similar articles
-
CoVaccine HT™ adjuvant is superior to Freund's adjuvants in eliciting antibodies against the endogenous alarmin HMGB1.J Immunol Methods. 2016 Dec;439:37-43. doi: 10.1016/j.jim.2016.09.008. Epub 2016 Sep 30. J Immunol Methods. 2016. PMID: 27693642
-
CoVaccine HT™ adjuvant is superior to Freund's in eliciting ovine polyclonal antibodies against human tumor necrosis factor-alpha.Adv Protein Chem Struct Biol. 2022;129:189-213. doi: 10.1016/bs.apcsb.2021.11.009. Epub 2022 Feb 16. Adv Protein Chem Struct Biol. 2022. PMID: 35305719
-
Recombinant influenza A virus hemagglutinin HA2 subunit protects mice against influenza A(H7N9) virus infection.Arch Virol. 2015 Mar;160(3):777-86. doi: 10.1007/s00705-014-2314-x. Epub 2015 Jan 24. Arch Virol. 2015. PMID: 25616843
-
Antibody Responsiveness to Influenza: What Drives It?Viruses. 2021 Jul 19;13(7):1400. doi: 10.3390/v13071400. Viruses. 2021. PMID: 34372607 Free PMC article. Review.
-
An indirect comparison meta-analysis of AS03 and MF59 adjuvants in pandemic influenza A(H1N1)pdm09 vaccines.Vaccine. 2019 Jul 18;37(31):4246-4255. doi: 10.1016/j.vaccine.2019.06.039. Epub 2019 Jun 26. Vaccine. 2019. PMID: 31253447 Review.
Cited by
-
Preserved antiviral adaptive immunity following polyclonal antibody immunotherapy for severe murine influenza infection.Sci Rep. 2016 Jul 6;6:29154. doi: 10.1038/srep29154. Sci Rep. 2016. PMID: 27380890 Free PMC article.
-
CoVaccine HT™ Adjuvant Potentiates Robust Immune Responses to Recombinant SARS-CoV-2 Spike S1 Immunization.Front Immunol. 2020 Oct 30;11:599587. doi: 10.3389/fimmu.2020.599587. eCollection 2020. Front Immunol. 2020. PMID: 33193454 Free PMC article.
-
Development of a Cost-effective Ovine Polyclonal Antibody-Based Product, EBOTAb, to Treat Ebola Virus Infection.J Infect Dis. 2016 Apr 1;213(7):1124-33. doi: 10.1093/infdis/jiv565. Epub 2015 Dec 28. J Infect Dis. 2016. PMID: 26715676 Free PMC article.
References
-
- McMillin GA, Owen WE, Lambert TL, De BK, Frank EL, et al. (2002) Comparable effects of DIGIBIND and DigiFab in thirteen digoxin immunoassays. Clin Chem 48: 1580–1584. - PubMed
-
- Redwan el RM, Fahmy A, El Hanafy A, Abd El-Baky N, Sallam SM (2009) Ovine anti-rabies antibody production and evaluation. Comp Immunol Microbiol Infect Dis 32: 9–19. - PubMed
-
- Sjostrom L, al-Abdulla IH, Rawat S, Smith DC, Landon J (1994) A comparison of ovine and equine antivenoms. Toxicon 32: 427–433. - PubMed
-
- Newcombe C, Newcombe AR (2007) Antibody production: polyclonal-derived biotherapeutics. J Chromatogr B Analyt Technol Biomed Life Sci 848: 2–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

