Mechanisms of talin-dependent integrin signaling and crosstalk
- PMID: 23891718
- PMCID: PMC3877210
- DOI: 10.1016/j.bbamem.2013.07.017
Mechanisms of talin-dependent integrin signaling and crosstalk
Abstract
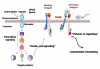
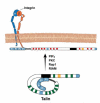
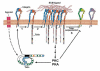
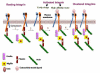
Cells undergo dynamic remodeling of the cytoskeleton during adhesion and migration on various extracellular matrix (ECM) substrates in response to physiological and pathological cues. The major mediators of such cellular responses are the heterodimeric adhesion receptors, the integrins. Extracellular or intracellular signals emanating from different signaling cascades cause inside-out signaling of integrins via talin, a cystokeletal protein that links integrins to the actin cytoskeleton. Various integrin subfamilies communicate with each other and growth factor receptors under diverse cellular contexts to facilitate or inhibit various integrin-mediated functions. Since talin is an essential mediator of integrin activation, much of the integrin crosstalk would therefore be influenced by talin. However, despite the existence of an extensive body of knowledge on the role of talin in integrin activation and as a stabilizer of ECM-actin linkage, information on its role in regulating inter-integrin communication is limited. This review will focus on the structure of talin, its regulation of integrin activation and discuss its potential role in integrin crosstalk. This article is part of a Special Issue entitled: Reciprocal influences between cell cytoskeleton and membrane channels, receptors and transporters. Guest Editor: Jean Claude Hervé.
Keywords: Actin; Crosstalk; Integrin; Talin.
© 2013.
Figures
Similar articles
-
Talin and signaling through integrins.Methods Mol Biol. 2012;757:325-47. doi: 10.1007/978-1-61779-166-6_20. Methods Mol Biol. 2012. PMID: 21909921 Free PMC article.
-
An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton.Nat Cell Biol. 2006 Jun;8(6):601-6. doi: 10.1038/ncb1411. Epub 2006 Apr 30. Nat Cell Biol. 2006. PMID: 16648844
-
Talin Autoinhibition Regulates Cell-ECM Adhesion Dynamics and Wound Healing In Vivo.Cell Rep. 2018 Nov 27;25(9):2401-2416.e5. doi: 10.1016/j.celrep.2018.10.098. Cell Rep. 2018. PMID: 30485809
-
Talin as a mechanosensitive signaling hub.J Cell Biol. 2018 Nov 5;217(11):3776-3784. doi: 10.1083/jcb.201808061. Epub 2018 Sep 25. J Cell Biol. 2018. PMID: 30254032 Free PMC article. Review.
-
The talin wags the dog: new insights into integrin activation.Trends Cell Biol. 2004 Feb;14(2):55-7. doi: 10.1016/j.tcb.2003.12.009. Trends Cell Biol. 2004. PMID: 15106607 Review.
Cited by
-
WAVE2 Regulates Actin-Dependent Processes Induced by the B Cell Antigen Receptor and Integrins.Cells. 2023 Nov 25;12(23):2704. doi: 10.3390/cells12232704. Cells. 2023. PMID: 38067132 Free PMC article.
-
Exogenous Integrin αIIbβ3 Inhibitors Revisited: Past, Present and Future Applications.Int J Mol Sci. 2021 Mar 25;22(7):3366. doi: 10.3390/ijms22073366. Int J Mol Sci. 2021. PMID: 33806083 Free PMC article. Review.
-
The structural basis of β2 integrin intra-cellular multi-protein complexes.Biophys Rev. 2022 Sep 7;14(5):1183-1195. doi: 10.1007/s12551-022-00995-x. eCollection 2022 Oct. Biophys Rev. 2022. PMID: 36345283 Free PMC article. Review.
-
Phosphoproteomic Profiling Identifies Aberrant Activation of Integrin Signaling in Aggressive Non-Type Bladder Carcinoma.J Clin Med. 2019 May 17;8(5):703. doi: 10.3390/jcm8050703. J Clin Med. 2019. PMID: 31108958 Free PMC article.
-
Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis.Nat Rev Rheumatol. 2020 Apr;16(4):193-207. doi: 10.1038/s41584-019-0364-x. Epub 2020 Feb 20. Nat Rev Rheumatol. 2020. PMID: 32080619 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

