Review series: Rab GTPases and membrane identity: causal or inconsequential?
- PMID: 23878272
- PMCID: PMC3718981
- DOI: 10.1083/jcb.201306010
Review series: Rab GTPases and membrane identity: causal or inconsequential?
Abstract
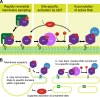
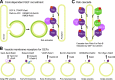
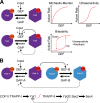
Rab GTPases are highly conserved components of vesicle trafficking pathways that help to ensure the fusion of a vesicle with a specific target organelle membrane. Specific regulatory pathways promote kinetic proofreading of membrane surfaces by Rab GTPases, and permit accumulation of active Rabs only at the required sites. Emerging evidence indicates that Rab activation and inactivation are under complex feedback control, suggesting that ultrasensitivity and bistability, principles established for other cellular regulatory networks, may also apply to Rab regulation. Such systems can promote the rapid membrane accumulation and removal of Rabs to create time-limited membrane domains with a unique composition, and can explain how Rabs define the identity of vesicle and organelle membranes.
Figures
Similar articles
-
Rab proteins of the endoplasmic reticulum: functions and interactors.Biochem Soc Trans. 2012 Dec 1;40(6):1426-32. doi: 10.1042/BST20120158. Biochem Soc Trans. 2012. PMID: 23176493 Review.
-
Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases.J Biol Chem. 2013 Oct 4;288(40):28704-12. doi: 10.1074/jbc.M113.488213. Epub 2013 Aug 26. J Biol Chem. 2013. PMID: 23979137 Free PMC article.
-
Rab family of small GTPases: an updated view on their regulation and functions.FEBS J. 2021 Jan;288(1):36-55. doi: 10.1111/febs.15453. Epub 2020 Jul 1. FEBS J. 2021. PMID: 32542850 Free PMC article. Review.
-
Rab GTPase localization and Rab cascades in Golgi transport.Biochem Soc Trans. 2012 Dec 1;40(6):1373-7. doi: 10.1042/BST20120168. Biochem Soc Trans. 2012. PMID: 23176483 Free PMC article. Review.
-
Rab regulation by GEFs and GAPs during membrane traffic.Curr Opin Cell Biol. 2019 Aug;59:34-39. doi: 10.1016/j.ceb.2019.03.004. Epub 2019 Apr 10. Curr Opin Cell Biol. 2019. PMID: 30981180 Review.
Cited by
-
Spatial and Functional Aspects of ER-Golgi Rabs and Tethers.Front Cell Dev Biol. 2016 Apr 18;4:28. doi: 10.3389/fcell.2016.00028. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27148530 Free PMC article.
-
Recycling Endosomes and Viral Infection.Viruses. 2016 Mar 8;8(3):64. doi: 10.3390/v8030064. Viruses. 2016. PMID: 27005655 Free PMC article. Review.
-
EHBP1L1 coordinates Rab8 and Bin1 to regulate apical-directed transport in polarized epithelial cells.J Cell Biol. 2016 Feb 1;212(3):297-306. doi: 10.1083/jcb.201508086. J Cell Biol. 2016. PMID: 26833786 Free PMC article.
-
Regulation of post-Golgi LH3 trafficking is essential for collagen homeostasis.Nat Commun. 2016 Jul 20;7:12111. doi: 10.1038/ncomms12111. Nat Commun. 2016. PMID: 27435297 Free PMC article.
-
The Atlastin C-terminal tail is an amphipathic helix that perturbs the bilayer structure during endoplasmic reticulum homotypic fusion.J Biol Chem. 2015 Feb 20;290(8):4772-4783. doi: 10.1074/jbc.M114.601823. Epub 2015 Jan 2. J Biol Chem. 2015. PMID: 25555915 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

