Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients ≤60 years with newly diagnosed acute myeloid leukemia
- PMID: 23877926
- PMCID: PMC4110914
- DOI: 10.1002/ajh.23544
Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients ≤60 years with newly diagnosed acute myeloid leukemia
Abstract
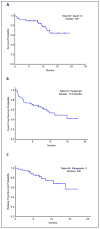
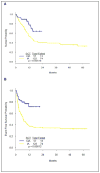
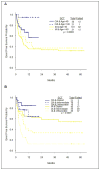
Clofarabine is a second generation nucleoside analogue with activity in adults with acute myeloid leukemia (AML). A phase I trial of clofarabine, idarubicin, and cytarabine (CIA) in relapsed and refractory AML had shown an overall response rate (ORR) of 48%. To explore this combination further, we conducted a phase II study of (CIA) in patients with newly diagnosed AML ≤60 years. Patients ≥18-60 years with AML and adequate organ function were enrolled. Induction therapy consisted of clofarabine (C) 20 mg m⁻² IV daily (days 1-5), idarubicin (I) 10 mg m⁻² IV daily (days 1-3), and cytarabine (A) 1 g m⁻² IV daily (days 1-5). Patients in remission received up to six consolidation cycles (C 15 mg m⁻² × 3, I 8 mg m⁻² × 2, and A 0.75 g m⁻² × 3). Fifty-seven patients were evaluable. ORR was 79%. With a median follow up of 10.9 months, the median overall survival (OS) was not reached, the median event-free survival (EFS) was 13.5 months. Most toxicities were ≤grade 2. Four week mortality was 2%. In subgroup analysis, patients ≤40 years had better OS (P = 0.04) and EFS (P = 0.04) compared to patients >40 years. Compared to historical patients treated with idarubicin and cyarabine (IA), the OS and EFS were significantly longer for CIA treated patients. In multivariate analysis, CIA retained its favorable impact on OS compared to IA. Thus, CIA is an effective and safe therapy for patients ≤60 years with newly diagnosed AML.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
Similar articles
-
A randomized phase 2 study of idarubicin and cytarabine with clofarabine or fludarabine in patients with newly diagnosed acute myeloid leukemia.Cancer. 2017 Nov 15;123(22):4430-4439. doi: 10.1002/cncr.30883. Epub 2017 Jul 14. Cancer. 2017. PMID: 28708931 Free PMC article. Clinical Trial.
-
Clofarabine combinations as acute myeloid leukemia salvage therapy.Cancer. 2008 Oct 15;113(8):2090-6. doi: 10.1002/cncr.23816. Cancer. 2008. PMID: 18756533 Free PMC article. Clinical Trial.
-
Clofarabine plus low-dose cytarabine followed by clofarabine plus low-dose cytarabine alternating with decitabine in acute myeloid leukemia frontline therapy for older patients.Cancer. 2012 Sep 15;118(18):4471-7. doi: 10.1002/cncr.27429. Epub 2012 Jan 26. Cancer. 2012. PMID: 22282348 Free PMC article. Clinical Trial.
-
The role of Clofarabine in the treatment of adults with acute myeloid leukemia.Crit Rev Oncol Hematol. 2015 Mar;93(3):237-45. doi: 10.1016/j.critrevonc.2014.10.009. Epub 2014 Oct 22. Crit Rev Oncol Hematol. 2015. PMID: 25457773 Review.
-
Clofarabine in the treatment of newly diagnosed acute myeloid leukemia in older adults.Ann Pharmacother. 2012 Jan;46(1):89-96. doi: 10.1345/aph.1Q295. Epub 2011 Dec 13. Ann Pharmacother. 2012. PMID: 22170975 Review.
Cited by
-
Treatment with Hypomethylating Agents before Allogeneic Stem Cell Transplant Improves Progression-Free Survival for Patients with Chronic Myelomonocytic Leukemia.Biol Blood Marrow Transplant. 2016 Jan;22(1):47-53. doi: 10.1016/j.bbmt.2015.08.031. Epub 2015 Sep 4. Biol Blood Marrow Transplant. 2016. PMID: 26343946 Free PMC article. Clinical Trial.
-
Characterization of oral and gut microbiome temporal variability in hospitalized cancer patients.Genome Med. 2017 Feb 28;9(1):21. doi: 10.1186/s13073-017-0409-1. Genome Med. 2017. PMID: 28245856 Free PMC article.
-
Peripheral blood blast clearance is an independent prognostic factor for survival and response to acute myeloid leukemia induction chemotherapy.Am J Hematol. 2016 Dec;91(12):1221-1226. doi: 10.1002/ajh.24500. Epub 2016 Aug 29. Am J Hematol. 2016. PMID: 27474808 Free PMC article.
-
A randomized phase 2 study of idarubicin and cytarabine with clofarabine or fludarabine in patients with newly diagnosed acute myeloid leukemia.Cancer. 2017 Nov 15;123(22):4430-4439. doi: 10.1002/cncr.30883. Epub 2017 Jul 14. Cancer. 2017. PMID: 28708931 Free PMC article. Clinical Trial.
-
The role of clofarabine in acute myeloid leukemia.Leuk Lymphoma. 2013 Apr;54(4):688-98. doi: 10.3109/10428194.2012.726722. Epub 2012 Sep 28. Leuk Lymphoma. 2013. PMID: 22957815 Free PMC article. Review.
References
-
- Yates JW, Wallace HJ, Jr, Ellison RR, et al. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57:485–8. - PubMed
-
- Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58:1203–12. - PubMed
-
- O'Donnell MR, Abboud CN, Altman J, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2011;9:280–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical