Cell stress promotes the association of phosphorylated HspB1 with F-actin
- PMID: 23874834
- PMCID: PMC3707891
- DOI: 10.1371/journal.pone.0068978
Cell stress promotes the association of phosphorylated HspB1 with F-actin
Abstract
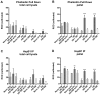
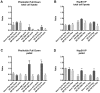
Previous studies have suggested that the small heat shock protein, HspB1, has a direct influence on the dynamics of cytoskeletal elements, in particular, filamentous actin (F-actin) polymerization. In this study we have assessed the influence of HspB1 phosphorylation on its interaction(s) with F-actin. We first determined the distribution of endogenous non-phosphorylated HspB1, phosphorylated HspB1 and F-actin in neuroendocrine PC12 cells by immunocytochemistry and confocal microscopy. We then investigated a potential direct interaction between HspB1 with F-actin by precipitating F-actin directly with biotinylated phalloidin followed by Western analyses; the reverse immunoprecipitation of HspB1 was also carried out. The phosphorylation influence of HspB1 in this interaction was investigated by using pharmacologic inhibition of p38 MAPK. In control cells, HspB1 interacts with F-actin as a predominantly non-phosphorylated protein, but subsequent to stress there is a redistribution of HspB1 to the cytoskeletal fraction and a significantly increased association of pHspB1 with F-actin. Our data demonstrate HspB1 is found in a complex with F-actin both in phosphorylated and non-phosphorylated forms, with an increased association of pHspB1 with F-actin after heat stress. Overall, our study combines both cellular and biochemical approaches to show cellular localization and direct demonstration of an interaction between endogenous HspB1 and F-actin using methodolgy that specifically isolates F-actin.
Conflict of interest statement
Figures
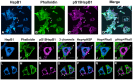
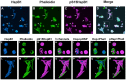
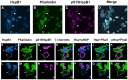


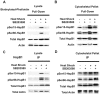
Similar articles
-
Phosphorylation of the small heat shock protein HspB1 regulates cytoskeletal recruitment and cell motility.Mol Biol Cell. 2022 Sep 15;33(11):ar100. doi: 10.1091/mbc.E22-02-0057. Epub 2022 Jun 29. Mol Biol Cell. 2022. PMID: 35767320 Free PMC article.
-
Phosphorylation-dependent subcellular localization of the small heat shock proteins HspB1/Hsp25 and HspB5/αB-crystallin in cultured hippocampal neurons.Histochem Cell Biol. 2012 Sep;138(3):407-18. doi: 10.1007/s00418-012-0964-x. Epub 2012 May 23. Histochem Cell Biol. 2012. PMID: 22617993
-
HSPB1, actin filament dynamics, and aging cells.Ann N Y Acad Sci. 2010 Jun;1197:76-84. doi: 10.1111/j.1749-6632.2010.05191.x. Ann N Y Acad Sci. 2010. PMID: 20536836 Review.
-
The role of Hsp27 and actin in the regulation of movement in human cancer cells responding to heat shock.Cell Stress Chaperones. 2009 Sep;14(5):445-57. doi: 10.1007/s12192-008-0098-1. Epub 2009 Feb 18. Cell Stress Chaperones. 2009. PMID: 19224398 Free PMC article.
-
Mammalian HspB1 (Hsp27) is a molecular sensor linked to the physiology and environment of the cell.Cell Stress Chaperones. 2017 Jul;22(4):517-529. doi: 10.1007/s12192-017-0765-1. Epub 2017 Jan 31. Cell Stress Chaperones. 2017. PMID: 28144778 Free PMC article. Review.
Cited by
-
Structure and properties of chimeric small heat shock proteins containing yellow fluorescent protein attached to their C-terminal ends.Cell Stress Chaperones. 2014 Jul;19(4):507-18. doi: 10.1007/s12192-013-0477-0. Epub 2013 Nov 27. Cell Stress Chaperones. 2014. PMID: 24282123 Free PMC article.
-
DNAJA1‑knockout alleviates heat stroke‑induced endothelial barrier disruption via improving thermal tolerance and suppressing the MLCK‑MLC signaling pathway.Mol Med Rep. 2024 May;29(5):87. doi: 10.3892/mmr.2024.13211. Epub 2024 Mar 29. Mol Med Rep. 2024. PMID: 38551163 Free PMC article.
-
A peptide-crosslinking approach identifies HSPA8 and PFKL as selective interactors of an actin-derived peptide containing reduced and oxidized methionine.RSC Chem Biol. 2022 Sep 15;3(10):1282-1289. doi: 10.1039/d2cb00183g. eCollection 2022 Oct 5. RSC Chem Biol. 2022. PMID: 36320891 Free PMC article.
-
Phosphoserine-86-HSPB1 (pS86-HSPB1) is cytoplasmic and highly induced in rat myometrium at labour.Histochem Cell Biol. 2023 Feb;159(2):149-162. doi: 10.1007/s00418-022-02158-1. Epub 2022 Oct 19. Histochem Cell Biol. 2023. PMID: 36260112 Free PMC article.
-
Heat shock protein 27 phosphorylation state is associated with cancer progression.Front Genet. 2014 Oct 6;5:346. doi: 10.3389/fgene.2014.00346. eCollection 2014. Front Genet. 2014. PMID: 25339975 Free PMC article. Review.
References
-
- Watson DF, Nachtman FN, Kuncl RW, Griffin JW (1994) Altered neurofilament phosphorylation and beta tubulin isotypes in Charcot-Marie-Tooth disease type 1. Neurology 44: 2383–2387. - PubMed
-
- Hsiao VC, Tian R, Long H, Der Perng M, Brenner M, et al. (2005) Alexander-disease mutation of GFAP causes filament disorganization and decreased solubility of GFAP. Journal of Cell Science 118: 2057–2065. - PubMed
-
- Fabrizi GM, Cavallaro T, Angiari C, Cabrini I, Taioli F, et al. (2007) Charcot–Marie–Tooth disease type 2E, a disorder of the cytoskeleton. Brain 130: 394–403. - PubMed
-
- Nollen EAA, Kabakov AE, Brunsting JF, Kanon B, Höhfeld J, et al. (2001) Modulation of in Vivo HSP70 Chaperone Activity by Hip and Bag-1. Journal of Biological Chemistry 276: 4677–4682. - PubMed
-
- Okada M, Hatakeyama T, Itoh H, Tokuta N, Tokumitsu H, et al. (2004) S100A1 Is a Novel Molecular Chaperone and a Member of the Hsp70/Hsp90 Multichaperone Complex. Journal of Biological Chemistry 279: 4221–4233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

