The COP9 signalosome is required for autophagy, proteasome-mediated proteolysis, and cardiomyocyte survival in adult mice
- PMID: 23873473
- PMCID: PMC3835345
- DOI: 10.1161/CIRCHEARTFAILURE.113.000338
The COP9 signalosome is required for autophagy, proteasome-mediated proteolysis, and cardiomyocyte survival in adult mice
Abstract
Background: The COP9 signalosome (CSN) is an evolutionarily conserved protein complex composed of 8 unique protein subunits (CSN1 through CSN8). We have recently discovered in perinatal mouse hearts that CSN regulates not only proteasome-mediated proteolysis but also macroautophagy. However, the physiological significance of CSN in a post-mitotic organ of adult vertebrates has not been determined. We sought to study the physiological role of CSN8/CSN in adult mouse hearts.
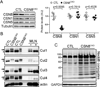
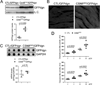
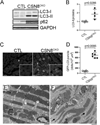
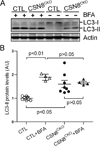
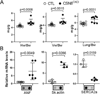
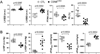
Methods and results: Csn8 was conditionally ablated in the cardiomyocytes of adult mice (CSN8(CKO)) using a temporally controlled Cre-LoxP system. Loss of CSN8 accumulated the neddylated forms of cullins and noncullin proteins, increased ubiquitinated proteins, and stabilized a surrogate substrate of the proteasome in the heart. Autophagic flux was significantly decreased, whereas autophagosomes were markedly increased in CSN8(CKO) hearts, indicative of impaired autophagosome removal. Furthermore, we observed increased oxidized proteins, massive necrotic cardiomyocytes, and morphological and functional changes characteristic of dilated cardiomyopathy in CSN8(CKO) mice.
Conclusions: CSN deneddylates substrates more than cullins and is indispensable to cardiomyocyte survival in not only perinatal hearts but also adult hearts. CSN8/CSN regulates both proteasome-mediated proteolysis and the autophagic-lysosomal pathway, critical to the removal of oxidized proteins in the heart.
Keywords: COP9 signalosome; NEDD8; autophagy; heart; proteasome endopeptidase complex.
Figures

Similar articles
-
COP9 signalosome controls the degradation of cytosolic misfolded proteins and protects against cardiac proteotoxicity.Circ Res. 2015 Nov 6;117(11):956-66. doi: 10.1161/CIRCRESAHA.115.306783. Epub 2015 Sep 17. Circ Res. 2015. PMID: 26383969 Free PMC article.
-
Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice.Circ Res. 2011 Jan 7;108(1):40-50. doi: 10.1161/CIRCRESAHA.110.230607. Epub 2010 Nov 4. Circ Res. 2011. PMID: 21051661 Free PMC article.
-
COP9 signalosome regulates autophagosome maturation.Circulation. 2011 Nov 8;124(19):2117-28. doi: 10.1161/CIRCULATIONAHA.111.048934. Epub 2011 Oct 10. Circulation. 2011. PMID: 21986281 Free PMC article.
-
Roles of COP9 signalosome in cancer.Cell Cycle. 2011 Sep 15;10(18):3057-66. doi: 10.4161/cc.10.18.17320. Epub 2011 Sep 15. Cell Cycle. 2011. PMID: 21876386 Free PMC article. Review.
-
Deregulation of the COP9 signalosome-cullin-RING ubiquitin-ligase pathway: mechanisms and roles in urological cancers.Int J Biochem Cell Biol. 2013 Jul;45(7):1327-37. doi: 10.1016/j.biocel.2013.03.023. Epub 2013 Apr 10. Int J Biochem Cell Biol. 2013. PMID: 23583660 Review.
Cited by
-
Role of the COP9 Signalosome (CSN) in Cardiovascular Diseases.Biomolecules. 2019 Jun 5;9(6):217. doi: 10.3390/biom9060217. Biomolecules. 2019. PMID: 31195722 Free PMC article. Review.
-
The ciliary protein RPGRIP1L governs autophagy independently of its proteasome-regulating function at the ciliary base in mouse embryonic fibroblasts.Autophagy. 2018;14(4):567-583. doi: 10.1080/15548627.2018.1429874. Epub 2018 Feb 21. Autophagy. 2018. PMID: 29372668 Free PMC article.
-
COP9 signalosome controls the degradation of cytosolic misfolded proteins and protects against cardiac proteotoxicity.Circ Res. 2015 Nov 6;117(11):956-66. doi: 10.1161/CIRCRESAHA.115.306783. Epub 2015 Sep 17. Circ Res. 2015. PMID: 26383969 Free PMC article.
-
Neddylation and deneddylation in cardiac biology.Am J Cardiovasc Dis. 2014 Dec 29;4(4):140-58. eCollection 2014. Am J Cardiovasc Dis. 2014. PMID: 25628956 Free PMC article. Review.
-
The necessity of NEDD8/Rub1 for vitality and its association with mitochondria-derived oxidative stress.Redox Biol. 2020 Oct;37:101765. doi: 10.1016/j.redox.2020.101765. Epub 2020 Oct 20. Redox Biol. 2020. PMID: 33099217 Free PMC article. Review.
References
-
- Schlossarek S, Carrier L. The ubiquitin-proteasome system in cardiomyopathies. Curr Opin Cardiol. 2011;26:190–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

